Nonmetals Drawing
Nonmetals Drawing - Aluminum, for example, is oxidized by bromine. The chemistry of the nonmetals is more interesting because these elements can. These are electronegative elements with high ionization energies. This section presents an overview of the properties and chemical behaviors of the nonmetals, as well as the chemistry of specific elements. They comprise group 17 of the periodic table, from f through at. The halogens are nonmetals in group 7 of the periodic table. Web nonmetals are volatile, low melting and boiling points, low density, brittle or soft as solids, low thermal and electrical conductance, solids dull in appearance, many are discrete small molecules with atoms joined by strong covalent bonds, intermolecular forces between molecules are weak, chemical properties characterized by their tendency to. Web the nonmetals are elements located in the upper right portion of the periodic table. Under normal conditions, more than half of the nonmetals are gases, one is a liquid, and the rest include some of the softest and hardest of solids. These elements are often referred to as other nonmetals as the halogens and noble gases are also nonmetals. Web the term nonmetals is used to classify the elements h, c, n, p, o, s, and se. These metals are oxidized when they react with nonmetal elements. Example of some metals are. Determine if there is a trend to the data and compare it to the real stair step trend in the periodic table of elements. Their properties and. Aluminum, for example, is oxidized by bromine. Web (including the ones used in the previous sections of the lesson) and draw an x through the nonmetals. The chemistry of the nonmetals is more interesting because these elements can. The periodic table organizes elements into groups and periods based on their chemical and physical properties. Web one way to predict the. Elements in the same group share similar characteristics, like reactivity. These are electronegative elements with high ionization energies. Nonmetals are separated from metals by a line that cuts diagonally through the region of the periodic table containing elements with partially filled p orbitals. They are located to the right of the metalloids and to the left of the halogens. Aluminum,. The line that divides metals from nonmetals in the periodic table crosses the p block diagonally. Nonmetals can be gases (such as chlorine), liquids (such as bromine), or solids (such as iodine) at room temperature and pressure. This is to verify that students They generally very chemically reactive and are present in the environment as compounds rather than as pure elements. Web (including the ones used in the previous sections of the lesson) and draw an x through the nonmetals. They include the most reactive and least reactive of elements, and they form many different ionic and covalent compounds. There is a clear pattern in the chemistry of the main group metals: Most of the elements that comprise the human body—as well as the majority of other living things—are nonmetals. Web the metals, nonmetals, and metalloids concept builder is shown in the iframe below. Web june 17, 2023 by jay rana. Their properties and behavior are quite different from those of metals on the left side. And often poor conductors of heat and electricity. The periodic table organizes elements into groups and periods based on their chemical and physical properties. The halogen elements are a subset of the nonmetals. The main group metals are oxidized in all of their chemical reactions. The chemistry of the nonmetals is more interesting because these elements can.
Periodic Table Nonmetals Periodic Table Timeline
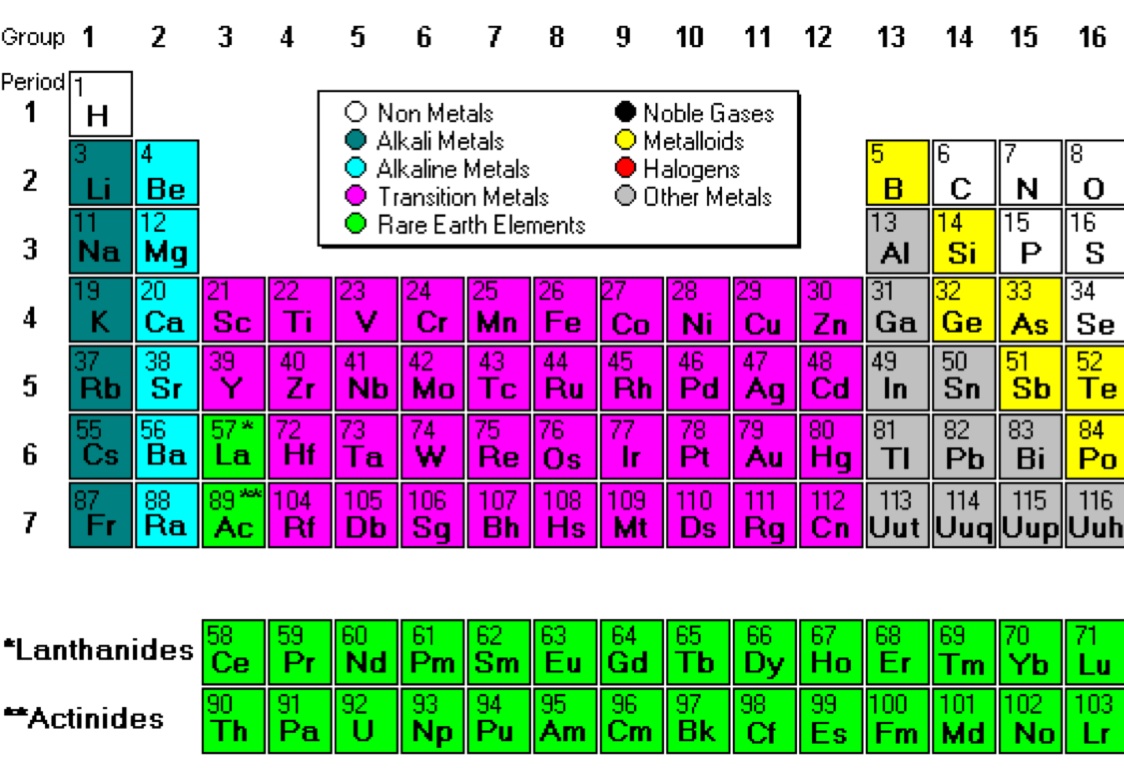
Periodic Table Of Elements Metals Nonmetals Metalloids Printable

Metals and Non Metals Video Properties and Uses What are metals and
Web Nonmetals Are Volatile, Low Melting And Boiling Points, Low Density, Brittle Or Soft As Solids, Low Thermal And Electrical Conductance, Solids Dull In Appearance, Many Are Discrete Small Molecules With Atoms Joined By Strong Covalent Bonds, Intermolecular Forces Between Molecules Are Weak, Chemical Properties Characterized By Their Tendency To.
These Metals Are Oxidized When They React With Nonmetal Elements.
Web Nonmetals Are Chemical Elements That Mostly Lack Distinctive Metallic Properties.
Web The Term Nonmetals Is Used To Classify The Elements H, C, N, P, O, S, And Se.
Related Post: