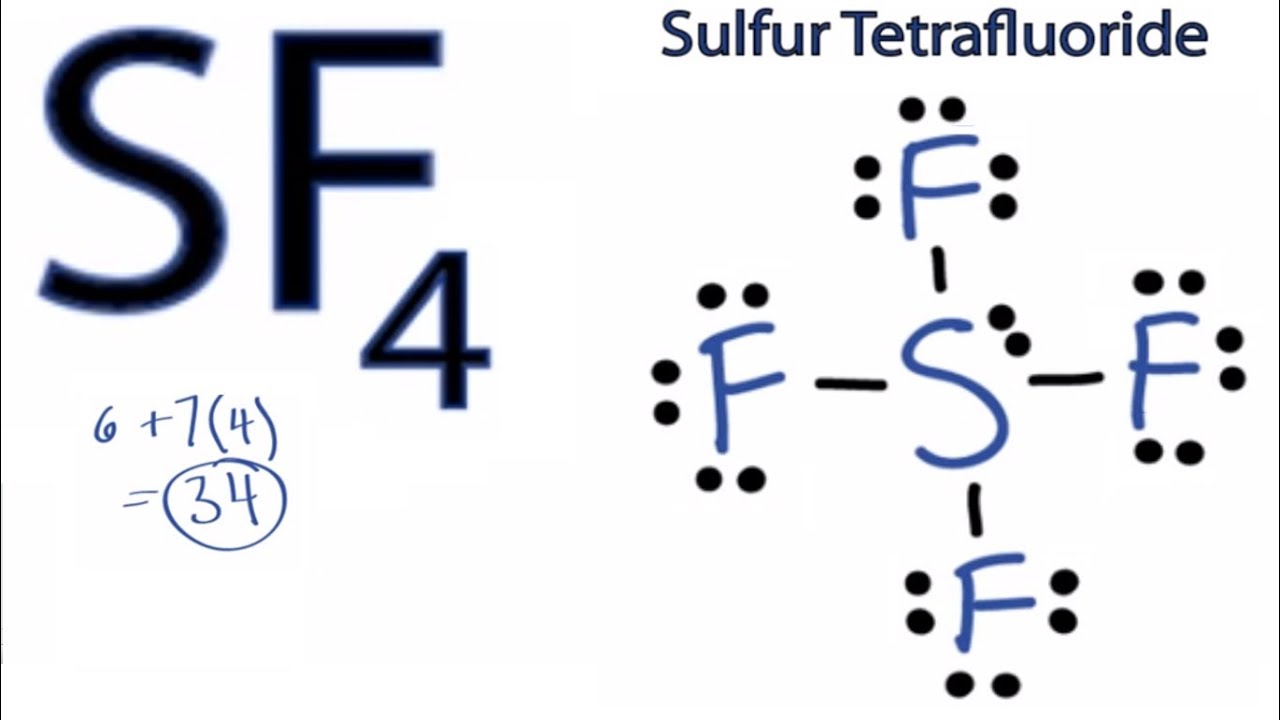
Draw The Lewis Structure For Sf4
Draw The Lewis Structure For Sf4 - Sulfur has a valence of 6. Remember that sulfur can hold more than 8 valence electrons. A video explanation of how to draw the lewis dot structure for sulfur tetrafluoride, along with. Web we draw lewis structures to predict: Draw the lewis structure for sf4 in the window below and then answer the questions that follow. #2 mention lone pairs on the atoms. Here, the given molecule is sf4. Web to draw the lewis structure for sf4, we start by determining the total number of valence electrons. What is the hybridization and formal charge on the sulfur? Web here’s how you can easily draw the sf 4 lewis structure step by step: Therefore sulfur becomes the center. A lewis structure is a way to show how atoms. Web to draw lewis structures for molecules and polyatomic ions with one central atom. Here, the given molecule is sf4. Web in sf4 lewis structure, each fluorine atom has joint with center sulfur atom. Web to draw lewis structures for molecules and polyatomic ions with one central atom. Web to draw the lewis structure for sf4, we start by determining the total number of valence electrons. In this structure sulfur will have ten valence electrons. 2.8k views 11 years ago chemistry lewis dot structures. Web in sf4 lewis structure, each fluorine atom has joint. What is the hybridization and formal charge on the sulfur? 2.8k views 11 years ago chemistry lewis dot structures. #2 mention lone pairs on the atoms. 100% (2 ratings) share share. Therefore sulfur becomes the center. Web we draw lewis structures to predict: What is the hybridization and formal charge on the sulfur? A lewis structure is a way to show how atoms. #1 draw a rough skeleton structure. Web to draw the sf4 lewis structure, follow these steps: Web for sf4 draw an appropriate lewis structure. 2.8k views 11 years ago chemistry lewis dot structures. Draw the lewis structure for sf4. Sulfur has a valence of 6. Web to draw the lewis structure for sf4, we start by determining the total number of valence electrons. Determine the total number of valence electrons by adding up the valence electrons of all atoms in the molecule. First, we arrange the atoms, then distribute the valence electrons, and finally, place them around the. Sulfur is in group 16 of the periodic table, so it has 6. Web 5 steps to draw the lewis structure of sf4 step #1: In this structure sulfur will have ten valence electrons. Sf 4 is lewis structure with sulfur (s).
How to draw Sf4 Lewis Structure? Beginners Guide

SF4 Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram
SF4 Lewis Structure How to Draw the Lewis Structure for SF4 YouTube
#2 Mention Lone Pairs On The Atoms.
Web Learn The Steps To Draw The Lewis Structure Of Sf4 In Just 1 Minute.📌You Can Draw Any Lewis Structures By Following The Simple Steps Mentioned In This Artic.
Calculate The Total Number Of Valence Electrons.
Here’s The Best Way To Solve It.
Related Post: