Draw The Structure Of Ethyl Propanoate
Draw The Structure Of Ethyl Propanoate - Identify the general structure for an ester. Use common names to name esters. Web what are the possible isomers of c5h10o2? Esters have the general formula rcoor ′,. Name esters according to the iupac system. For example, the molecular formula c 4 h 10 tells us there are 4 carbon atoms and 10 hydrogen atoms in a molecule, but it doesn’t distinguish between butane and isobutane. Web identify the general structure for an ester. Then attach the ethyl group to the bond that. Recognize that ethyl propanoate is an ester and recall the general structure of an ester, which involves a. C 5 h 10 o 2. Name esters according to the iupac system. Resonance, hybridization, lewis structures, orbitals: The alkyl group attached directly to the oxygen atom is a butyl group (in green). Web the part of the molecule derived from the carboxylic acid (in red) has three carbon atoms. Web what are the possible isomers of c5h10o2? Web we use several kinds of formulas to describe organic compounds. Web identify the general structure for an ester. Keeping in mind that the last carbon atom. Start with the portion from the acid. Resonance, hybridization, lewis structures, orbitals: One component is from the carboxylic acid (parent chain, which. Web identify the general structure for an ester. Use common names to name esters. Draw the resonance of ethyl propanoate. Resonance, hybridization, lewis structures, orbitals: Web identify the general structure for an ester. Resonance, hybridization, lewis structures, orbitals: Web draw the structure for each compound. Web 99% (94 ratings) share share. Keeping in mind that the last carbon atom. The part of the molecule derived from the carboxylic acid (in red) has three carbon. It is called propionate (common) or propanoate (iupac). Here’s how to approach this question. Web draw the pentanoate (five carbon atoms) group first; Then attach the ethyl group to the bond that. Use common names to name esters. Revision notes on isomerism of c5h10o2 molecules, isomerism in aliphatic carboxylic acids, isomerism in aliphatic esters,. Identify the general structure for an ester. For example, the molecular formula c 4 h 10 tells us there are 4 carbon atoms and 10 hydrogen atoms in a molecule, but it doesn’t distinguish between butane and isobutane. There are 2 steps to solve this one. Web draw one structure per sketcher.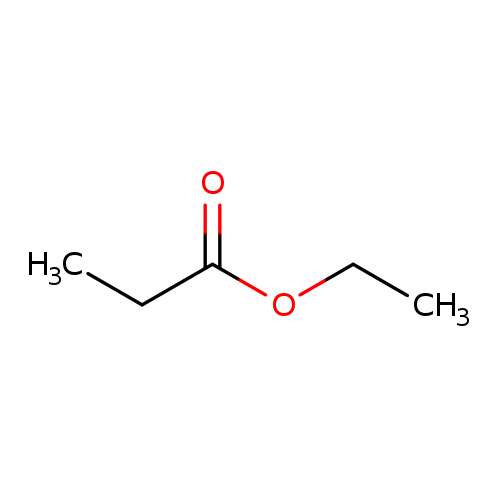
Structure Of Propanoate Lumen Learning
2ethyl propane Lewis dot structure Science, Chemistry, organic
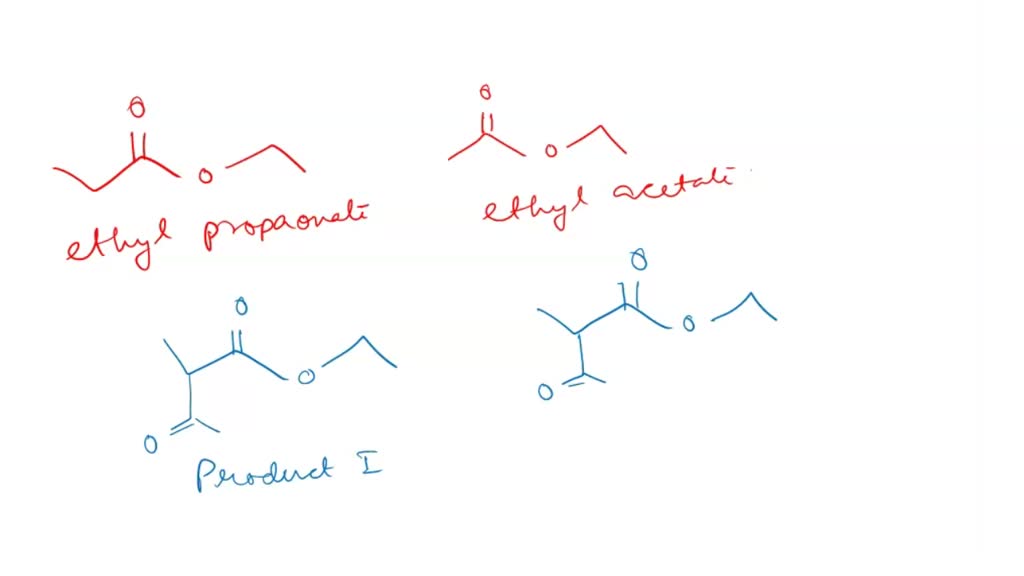
SOLVED When a 11 mixture of ethyl propanoate and ethyl acetate is
Recognize That Ethyl Propanoate Is An Ester And Recall The General Structure Of An Ester, Which Involves A.
Draw The Structure Of Ethyl Propanoate.
Draw The Pentanoate (Five Carbon Atoms) Group First;
Web What Are The Possible Isomers Of C5H10O2?
Related Post: