Draw The Lewis Dot Structure For Ch2Cl2
Draw The Lewis Dot Structure For Ch2Cl2 - #1 first draw a rough sketch. Carbon contributes 4 valence electrons, while each chlorine atom and each hydrogen atom contribute 7 and 1 valence electrons, respectively. Lewis structures can also be useful in predicting molecular geometry in. Draw the lewis dot structure for ch2cl2 below, showing approx. What is the electronic geometry, molecular geometry, and hybridization of c? Both hydrogen atoms and both chlorine atoms have made single bonds with carbon atom. Put our hydrogens here, and then our chlorines. First, determine the total number of valence electrons. Is the molecule polar or nonpolar? #3 calculate and mark formal charges on the atoms, if required. (c) what is the most important im force used by this molecule? Lewis structures can also be useful in predicting molecular geometry in. Let’s see how to do it. These are the constitutional isomers (drawn in lewis dot diagram notation) of c_2h_2cl_2, it's basically aligning the chlorine and hydrogen atoms in different places around the molecule. Ch 2 cl 2. This widget gets the lewis structure of chemical compounds. The number of dots equals the number of valence electrons in the atom. #3 calculate and mark formal charges on the atoms, if required. Let’s discuss each step in more detail. Web valence shell electrons of every atoms should be calculated first to determine the lewis dot structure of any molecule. #3 calculate and mark formal charges on the atoms, if required. Draw the lewis dot structure for ch2cl2 below, showing approx. Carbon is less electronegative than chlorine, so it'll go on the inside, and hydrogens always go on the outside. Is the molecule polar or nonpolar? Web what is the lewis structure of [//substance:ch2cl2//]? #1 first draw a rough sketch. Find more chemistry widgets in wolfram|alpha. Compute answers using wolfram's breakthrough technology & knowledgebase, relied on by millions of students & professionals. For the ch2cl2 structure use the periodic table to find the. For math, science, nutrition, history, geography, engineering, mathematics, linguistics, sports, finance, music…. Web use these steps to correctly draw the ch 2 cl 2 lewis structure: Let’s see how to do it. 7.7k views 1 year ago. Let’s discuss each step in more detail. Ch 2 cl 2 lewis structure. Each chlorine atom has three lone pairs and carbon atom does not has lone pairs. Begin by determining the total number of valence electrons in ch2cl2. Draw the lewis structure for ch2cl2. Carbon is less electronegative than chlorine, so it'll go on the inside, and hydrogens always go on the outside. Web to use the lewis structure calculator follow these steps: Enter the formula of the molecule in the field provided for it.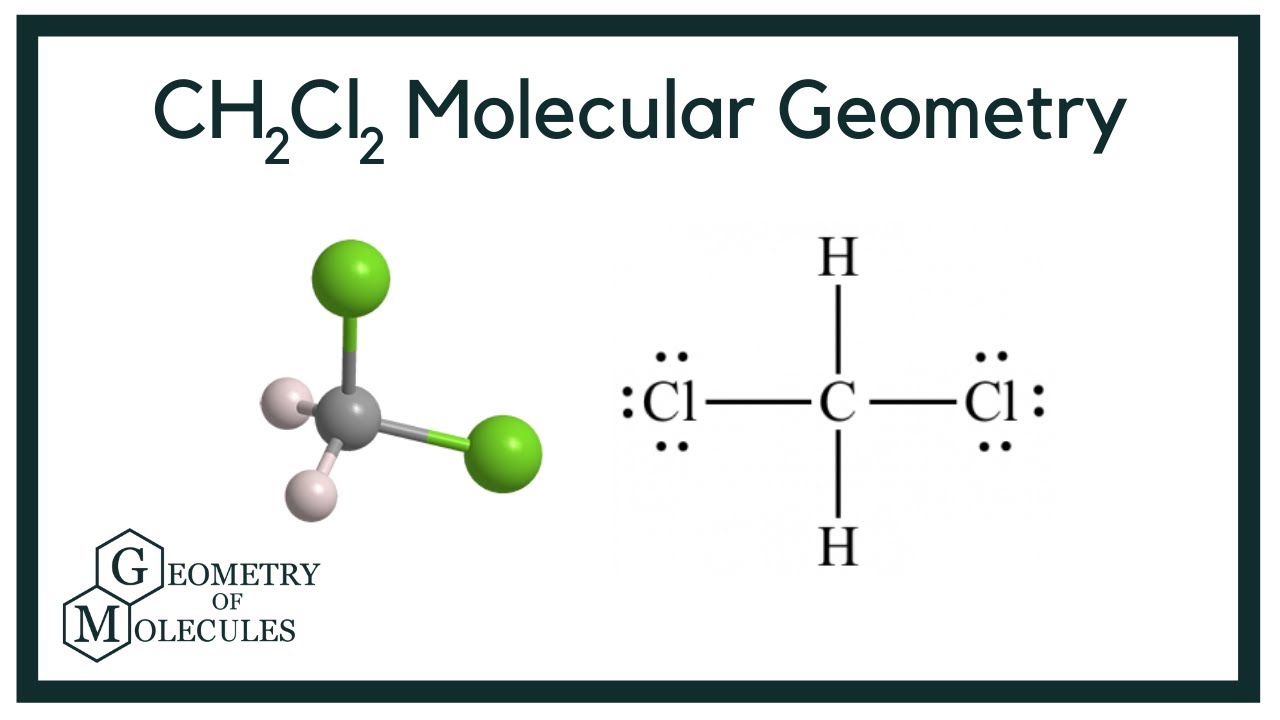
CH2Cl2 Molecular Geometry, Bond Angles & Electron Geometry

CH2Cl2 Lewis Structure How to Draw the Lewis Structure for CH2Cl2

How to draw CH2Cl2 Lewis Structure? Science Education and Tutorials
Lewis Structures Can Also Be Useful In Predicting Molecular Geometry In.
Send Feedback | Visit Wolfram|Alpha.
Carbon Contributes 4 Valence Electrons, While Each Chlorine Atom And Each Hydrogen Atom Contribute 7 And 1 Valence Electrons, Respectively.
Carbon (C) Contributes 4 Valence Electrons, Hydrogen (H) Has 1 Valence Electron, And Chlorine (Cl) Has 7 Valence Electrons Each.
Related Post: