Draw The Lewis Dot Structure For H2S
Draw The Lewis Dot Structure For H2S - Also, there are two lone pairs around sulfur atom. Web the lewis structure of h2s is as below. Web draw the lewis dot structure of hydrogen sulphide molecule. First, determine the total number of valence electrons. Assess the stability of a structure by considering formal charges of atoms. The sulfur atom (s) is at the center and it is surrounded by 2 hydrogen atoms (h). Web to draw the lewis structure of an atom, write the symbol of the atom and draw dots around it to represent the valence electrons. Web lewis structure of h2s contains single bonds between the sulfur (s) atom and each hydrogen (h) atom. Each step of drawing lewis structure of h 2 s is explained in detail in this tutorial. A lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. Also, there are two lone pairs around sulfur atom. Web to now determine the lewis structure of this molecule, we will first find out the total number of valence electrons for this molecule. First and foremost it is important to determine how many valence electrons are present in the compound. The sulfur atom (s) is at the center and it. A lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. 2 electrons (1 electron per atom) x 2 atoms = 2 electrons. Each step of drawing lewis structure of h 2 s is explained in detail in this tutorial. The number of dots equals the number of valence electrons in the atom. Web a. For example, here is the lewis structure for water, h 2 o. A lewis structure is a diagram that shows the chemical bonds between atoms in a molecule and the valence electrons or lone pairs of electrons. Hydrogen, group 1, has 1 valence electron, but we have two hydrogens here so let's multiply that by 2. Web let's do the. Web to draw the lewis structure of an atom, write the symbol of the atom and draw dots around it to represent the valence electrons. Count the total number of valence electrons. Web the lewis structure of hydrogen sulfide is easy to draw and understand. 40k views 2 years ago lewis structures. Each step of drawing lewis structure of h 2 s is explained in detail in this tutorial. Web a lewis electron dot diagram (or electron dot diagram, or a lewis diagram, or a lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. In this video, we go through the valence electrons for h2s. Web a lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: #2 next, indicate lone pairs on the atoms. Figure 7.9 lewis symbols illustrating the number of valence electrons for each element in the third period of the periodic table. A lewis structure is a diagram that shows the chemical bonds between atoms in a molecule and the valence electrons or lone pairs of electrons. For the h2s structure use the periodic table to find the total number of valence electrons. Valence electrons in hydrogen (h): To determine the number of valence electrons in hydrogen sulfide, add the number of valence electrons in each atom. The number of dots equals the number of valence electrons in the atom. Concept of number of total valence electrons of sulfur and hydrogen atoms are used to draw lewis structure of h 2 s.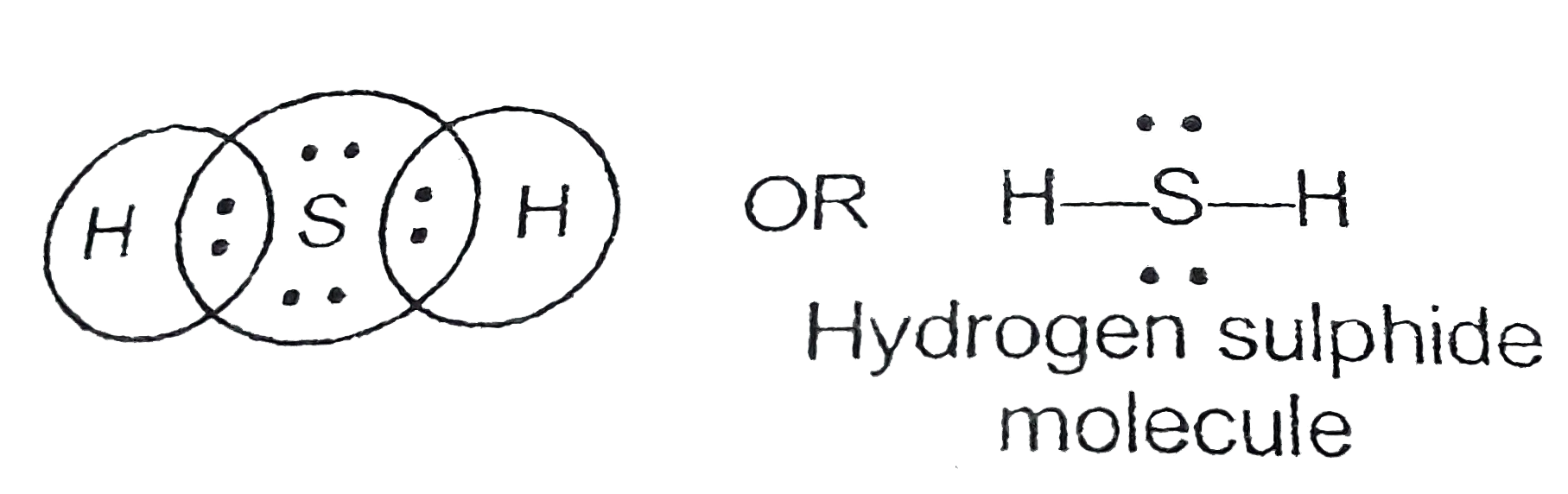
31+ H2S Lewis Structure Pictures Bepe Enthusiastic
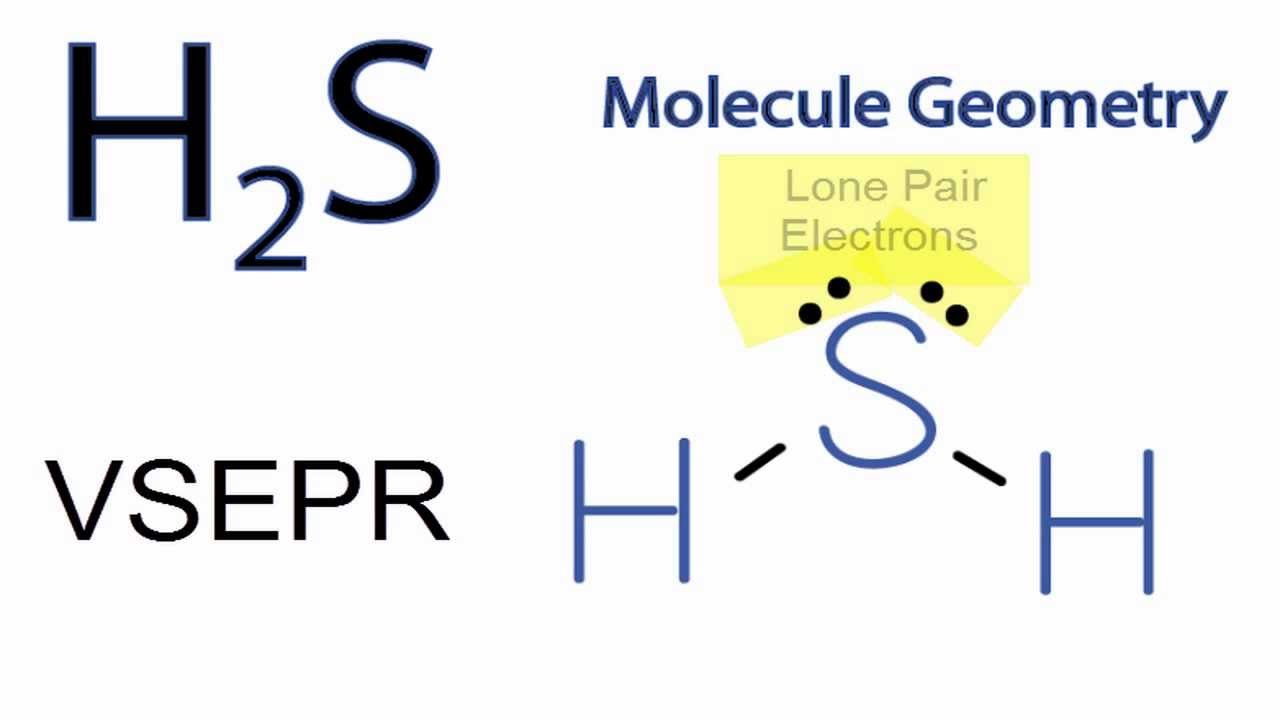
H2S Lewis Structure, Molecular Geometry, Hybridization and Polarity
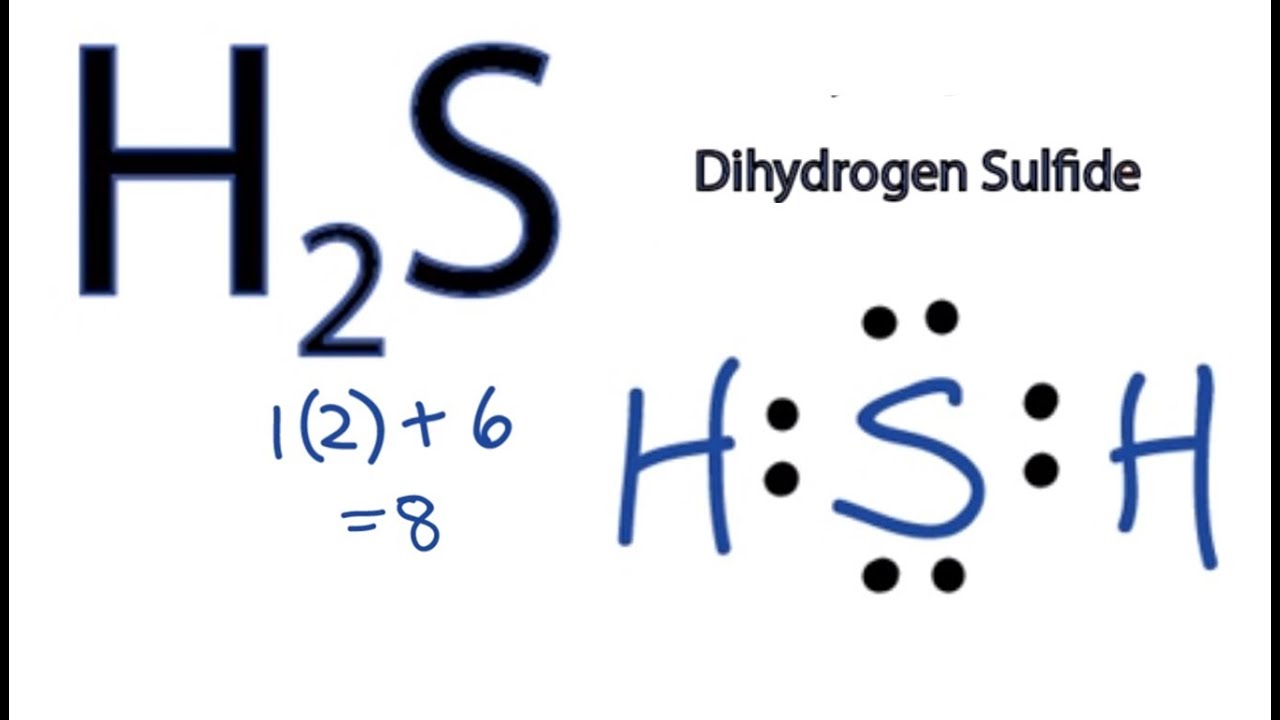
H2S Lewis Structure How to Draw the Dot Structure for H2S YouTube
Web Let's Do The Lewis Structure For H2S:
13K Views 3 Years Ago.
Web Lewis Structure Of H2S Contains Single Bonds Between The Sulfur (S) Atom And Each Hydrogen (H) Atom.
Web So Let's Say We Wanted To Draw The Dot Structure For This Molecule, So Silicon Tetrafluoride.
Related Post: