Water Molecule Drawing
Water Molecule Drawing - Web each water molecule links to four others creating a tetrahedral arrangement, however they are able to move freely and slide past each other, while ice forms a solid, larger hexagonal structure. Free water molecule illustrations to use in your next project. The one and only electron ring around the nucleus of each hydrogen atom has only one electron. The water molecule, visualized three different ways: Water is made up of two hydrogens and one oxygen atom, arranged in a tetrahedral shape. Each step of drawing lewis structure of h 2 o are. In the covalent bond between oxygen and hydrogen, the oxygen atom attracts electrons a bit more strongly than the. 64k views 4 years ago. Number of total valence electrons of oxygen and hydrogen atoms are used to draw lewis structure. The negative charge of the electron is balanced by the positive charge of one proton in the hydrogen nucleus. Number of total valence electrons of oxygen and hydrogen atoms are used to draw lewis structure. Once you’ve drawn a molecule, you can click the 2d to 3d button to convert the molecule into a 3d model which is then. Advanced materials, processes and energy devices , gated videos. This is because the oxygen atom, in addition to forming bonds. The configuration of the water molecule. Web a water molecule consists of two hydrogen atoms bonded to an oxygen atom, and its overall structure is bent. A quick explanation of the molecular geometry of h2o (water) including a description of the h2o bond angles.looking at the h2o lewis structure. 64k views 4 years ago. See how the two hydrogen atoms. The structural formula editor is surround by three toolbars which contain the tools you can use in the editor. Web each water molecule links to four others creating a tetrahedral arrangement, however they are able to move freely and slide past each other, while ice forms a solid, larger hexagonal structure. Each molecule is electrically neutral but polar, with the. In a water molecule, the oxygen atom and hydrogen atoms share electrons in covalent bonds, but the sharing is not equal. Web 14+ free water molecule illustrations. Web as water molecules make hydrogen bonds with each other, water takes on some unique chemical characteristics compared to other liquids and, since living things have a high water content, understanding these chemical features is key to understanding life. Advanced materials, processes and energy devices , gated videos. The bent shape of water. In the covalent bond between oxygen and hydrogen, the oxygen atom attracts electrons a bit more strongly than the. Explain what is meant by hydrogen bonding and the molecular structural features that bring it about. This is because the oxygen atom, in addition to forming bonds with the hydrogen atoms, also carries two pairs of unshared electrons. Each step of drawing lewis structure of h 2 o are. Number of total valence electrons of oxygen and hydrogen atoms are used to draw lewis structure. The structural formula editor is surround by three toolbars which contain the tools you can use in the editor. The one and only electron ring around the nucleus of each hydrogen atom has only one electron. The negative charge of the electron is balanced by the positive charge of one proton in the hydrogen nucleus. Web the water molecule, visualized three different ways: A quick explanation of the molecular geometry of h2o (water) including a description of the h2o bond angles.looking at the h2o lewis structure. Each molecule is electrically neutral but polar, with the center of positive and negative charges located in different places.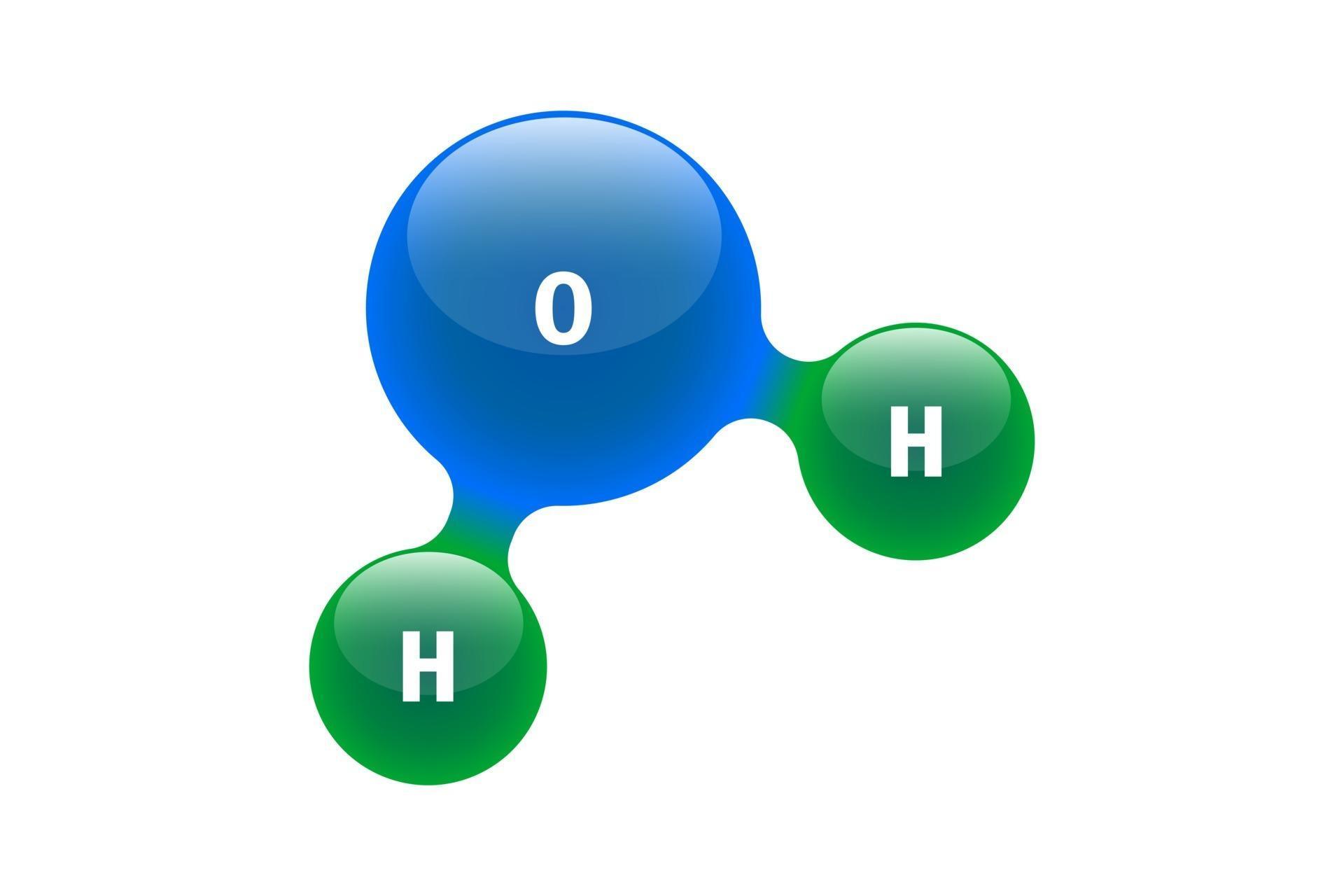
Chemistry model of molecule water H2O scientific elements. Integrated
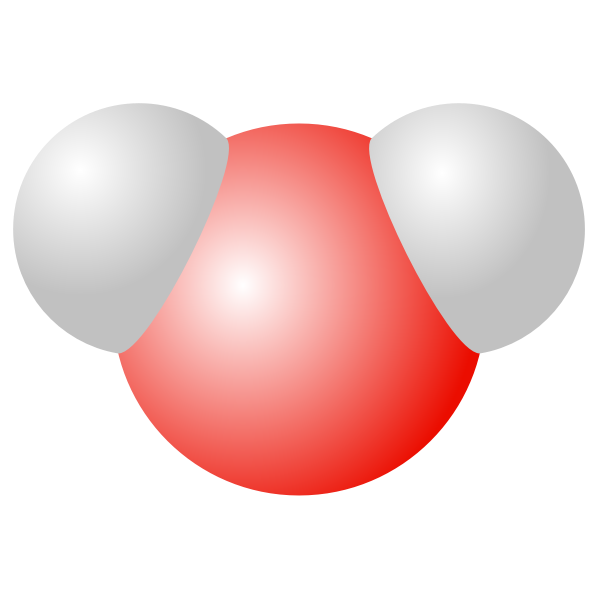
Water molecule vector drawing Free SVG

FileWater molecule 3D.svg Citizendium
There Are Two Lone Pairs Of Electrons On Each Oxygen Atom (Represented.
Water Is Made Up Of Two Hydrogens And One Oxygen Atom, Arranged In A Tetrahedral Shape.
The Configuration Of The Water Molecule.
Web Lewis Structure Of Water Molecule Contains Two Single Bonds Around Oxygen Atom.
Related Post: