Surface Tension Drawing
Surface Tension Drawing - These principles will be demonstrated by adding drops of different liquids to pennies to determine the strength of molecular attraction. Liquids with strong intermolecular forces have higher surface tensions than liquids with weaker forces. Since these intermolecular forces vary depending on the nature of the liquid (e.g. Surface tension keeps the paint from sinking (mostly). Surfactants like detergent), each solution exhibits differing surface tension properties. Gamma = f / d. Jackson’s aluminium painting panels are an exciting new surface for painting, suitable for all media. Explore surface tension and how it varies from one liquid to another. Water striders) to float on a water surface without becoming even partly submerged. Σ = f s / l (1) where. Distinguish between adhesive and cohesive forces. That means a drop of water will want to have the smallest possible surface area. View surface tension drawing videos. Water striders) to float on a water surface without becoming even partly submerged. The liquid film exerts a force on the movable wire in an attempt to reduce its surface area. Web water has a high surface tension because the water molecules on the surface are pulled together by strong hydrogen bonds. If the surface line element is a closed loop What is responsible for the strong surface tension in water? View surface tension drawing videos. The liquid film exerts a force on the movable wire in an attempt to reduce. Jackson’s aluminium painting panels are an exciting new surface for painting, suitable for all media. Describe the roles of intermolecular attractive forces in each of these properties/phenomena. Surfactants like detergent), each solution exhibits differing surface tension properties. The surface of the water is made up of millions of water molecules. The shape that has the smallest possible area for a. Surface tension can be defined as. What is responsible for the strong surface tension in water? Describe the roles of intermolecular attractive forces in each of these properties/phenomena. Gasoline) or solutes in the liquid (e.g. Surface tension drawing pictures, images and stock photos. Web water has a high surface tension because the water molecules on the surface are pulled together by strong hydrogen bonds. Web surface tension, denoted as γ, is defined as the force per unit of length that acts orthogonally to an imaginary line drawn on the interface, as shown in the diagram below. A surface line element d‘ will feel a total force σd‘ owing to the local surface tension σ(x). Surfactants like detergent), each solution exhibits differing surface tension properties. How do they differ from traditional supports? Surface tension is measured in si units of n/m (newton per meter), although the more common unit is the cgs unit dyn/cm (dyne per centimeter). Surfactants like detergent), each solution exhibits differing surface tension properties. Rearranging the equation to solve for surface tension yields s = f/2d. Distinguish between adhesive and cohesive forces. Gamma = f / d. Drawing & painting on aluminium.
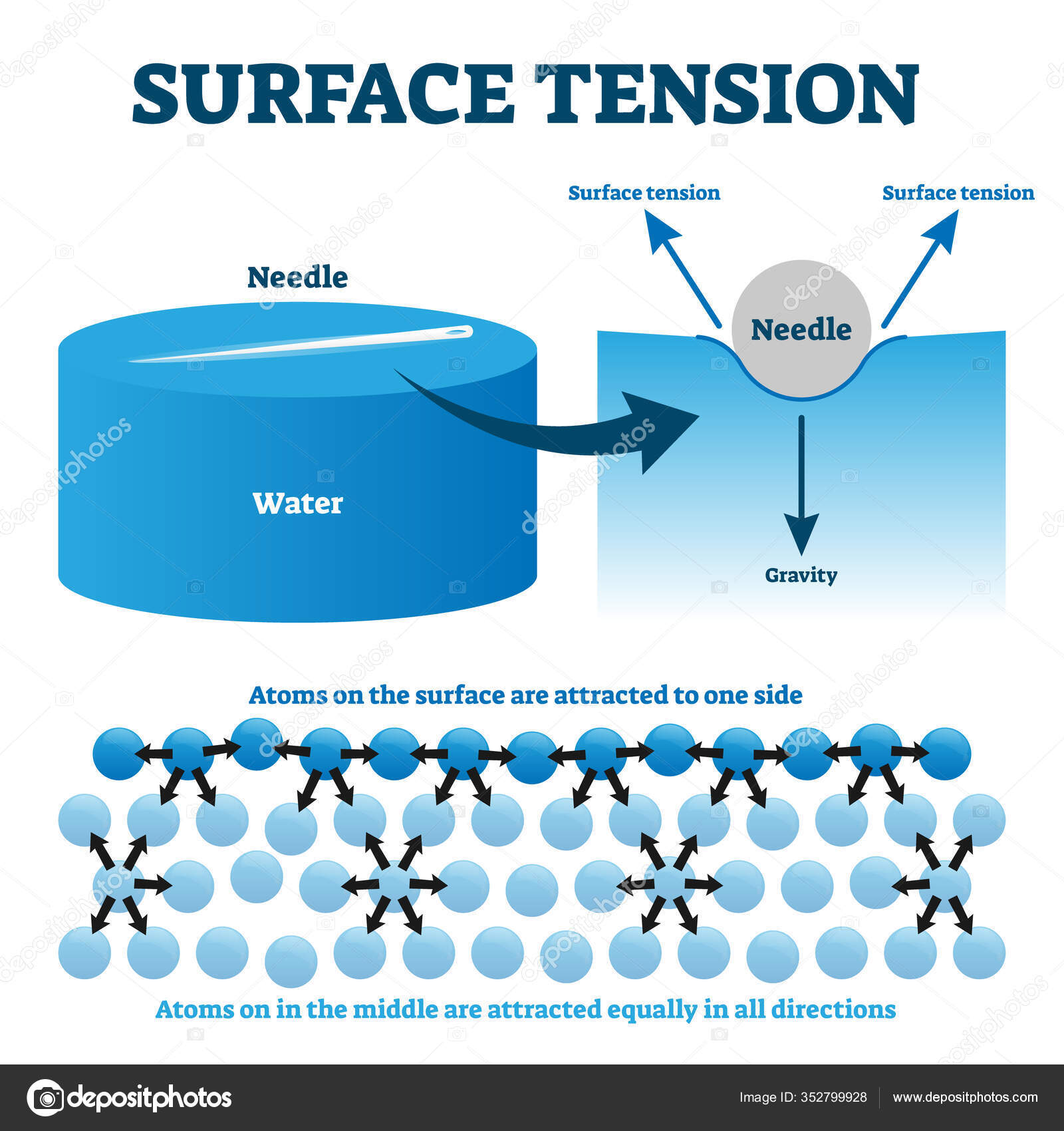
Explain the surface tension phenomenon with examples.

Surface Tension stock vector. Illustration of environment 92206295
Surface tension explanation vector illustration diagram Stock Vector
Σ = F S / L (1) Where.
That Means A Drop Of Water Will Want To Have The Smallest Possible Surface Area.
Water Striders) To Float On A Water Surface Without Becoming Even Partly Submerged.
The Cause Of Surface Tension Is Often Explained Roughly As Follows.
Related Post: