Specific Heat Drawing
Specific Heat Drawing - Specific heat for some common products are given in the table below. The units for specific heat can either be joules per gram per degree \(\left( \text{j/g}^\text{o} \text{c} \right)\) or calories per gram per degree \(\left( \text{cal/g}^\text{o} \text{c} \right)\). In addition, you will learn the formula that goes along with this concept and go through an example to work out the math. Specific heats of selected substances are given in the following table. Heat capacity is measured in j/ (kg·k). Web the symbol for specific heat is \(c_p\), with the \(p\) subscript referring to the fact that specific heats are measured at constant pressure. Digital device with spreadsheet program. Independent variable = time, t. Convection occurs when hot air. C = \frac {q} {m \delta t} c = mδt q. [7] where represents the amount of heat needed to uniformly raise the temperature of the sample by a small increment. Heat capacity is measured in j/ (kg·k). This means that if you had 1 kg of water, it would take 4190 j (joules) of heat energy to raise its temperature by 1. Web specific heat of common substances. The specific. There are three forms of thermal energy transfer: Web in this simulation, students will play the role of engineer in deciding which materials are the best candidates for a building project. Web specific heat (c) is the amount of heat required to change the temperature of a mass unit of a substance by one degree. Specific heat online unit converter.. Web specific heat (c) is the amount of heat required to change the temperature of a mass unit of a substance by one degree. The specific heat is the amount of heat necessary to change the temperature of 1.00 kg of mass by 1.00 ºc. Web 1 learn the fundamentals. Web the specific heat of a material is the amount. Specific heat, the quantity of heat required to raise the temperature of one gram of a substance by one celsius degree. The specific heat measures how much energy is stored in a heated object. Web the formula for specific heat looks like this: Web in this simulation, students will play the role of engineer in deciding which materials are the best candidates for a building project. [7] where represents the amount of heat needed to uniformly raise the temperature of the sample by a small increment. Q q is the amount of supplied or subtracted heat (in joules), m m is the mass of the sample, and \delta t δt is the difference between the initial and final temperatures. Heat capacity is measured in j/ (kg·k). The specific heat is the amount of heat necessary to change the temperature of 1.00 kg of mass by 1.00 ºc. Digital device with internet access. To determine the specific heat of a given solid specimen. Model the contents of a calorimeter as an isolated system to determine the unknown specific heat value of an unidentified metal. Specific heats of selected substances are given in the following table. An example is the specific heat of water, which is 4190 j/kg•c. Web the symbol c stands for specific heat, and depends on the material and phase. Why should we care about the specific heat? Its si unit is j/(kg ⋅ ⋅ k) or j/(kg ⋅ ⋅ °c °c).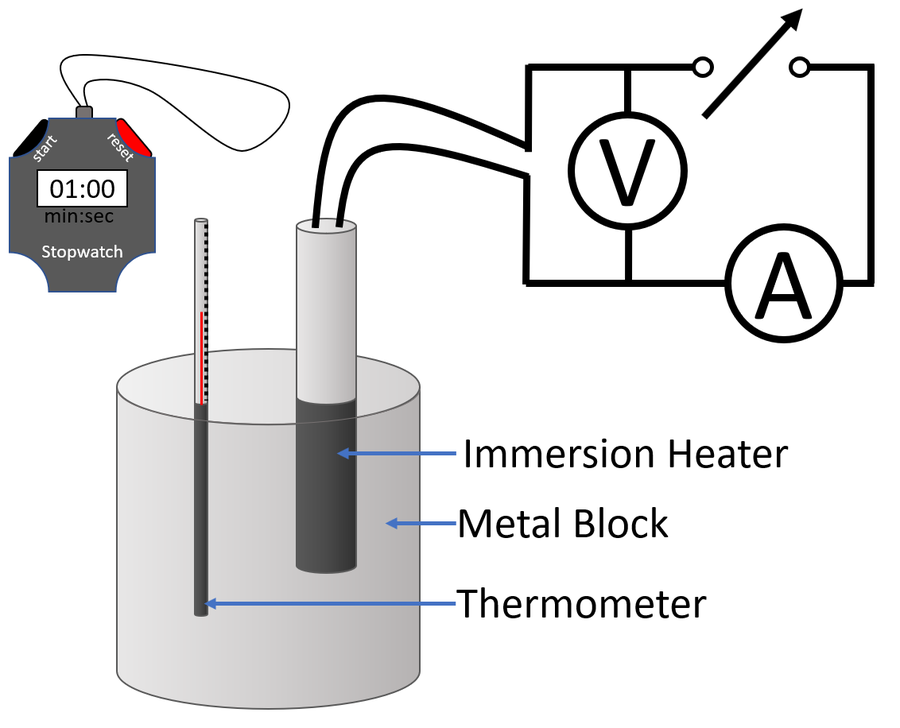
GCSE Physics Required Practical Determining Specific Heat Capacity
Specific Heat Definition Facts Britannica, 47 OFF

Lesson 10 Specific Heat YouTube
Specific Heat Online Unit Converter.
Web Every Substance Has A Characteristic Specific Heat, Which Is Reported In Units Of Cal/G•°C Or Cal/G•K, Depending On The Units Used To Express Δt.
This Means That If You Had 1 Kg Of Water, It Would Take 4190 J (Joules) Of Heat Energy To Raise Its Temperature By 1.
The Units For Specific Heat Can Either Be Joules Per Gram Per Degree \(\Left( \Text{J/G}^\Text{O} \Text{C} \Right)\) Or Calories Per Gram Per Degree \(\Left( \Text{Cal/G}^\Text{O} \Text{C} \Right)\).
Related Post: