Sigma Bond Drawing
Sigma Bond Drawing - 9.4 sigma and pi bonds | general chemistry. As illustrations, consider the bonds that have already been studied. The formation of a pi bond from p. With this information, you can easily count sigma and pi. After completing this section, you should be able to. Covalent bonds are formed by overlapping atomic orbitals, resulting in sigma and pi bonds. The diagram below (figure 3) is a representation of the energy levels of the bonding and antibonding orbitals formed in the hydrogen molecule. Web drawing lewis structures with multiple bonds. Various bond parameters such as bond length, bond angle, and bond enthalpy depend on the way the overlapping of atomic orbital takes place. This is a pi bond. Describe the formation of covalent bonds in terms of molecular orbitals. Π bonds are only found within double and triple bonds. The electron density is concentrated on opposite sides of the bond axis. Web the first bond between two atoms is always a sigma bond and the other bonds are always pi bonds. Web drawing lewis structures with multiple bonds. Various bond parameters such as bond length, bond angle, and bond enthalpy depend on the way the overlapping of atomic orbital takes place. This is the strongest form of covalent bonds, and this'll be a good basis for discussion maybe in the next video when we talk a little bit about pi bonds. This is a pi bond. Web how. The pi bond is the second bond of the double bonds between the carbon atoms, and is shown as an elongated green lobe that extends both above and below the plane of the molecule. Web the first bond between two atoms is always a sigma bond and the other bonds are always pi bonds. Web how do you count sigma. Π bonds are only found within double and triple bonds. Web draw an mo cartoon of a sigma bonding orbital formed by the overlap of two p orbitals between two oxygen atoms. To start, we must explain both bonds: Likewise, a triple bond consists of one sigma bond and two pi bonds. Web how do you count sigma and pi bonds? Web drawing lewis structures with multiple bonds. Web the figure below illustrates the sigma and pi bonds in an ethylene molecule (\(c_2h_4\)). * in a triple bond. The pi bond is the second bond of the double bonds between the carbon atoms, and is shown as an elongated green lobe that extends both above and below the plane of the molecule. Combining atomic orbitals, sigma and pi bonding. Sigma (σ) and pi (π). The diagram below (figure 3) is a representation of the energy levels of the bonding and antibonding orbitals formed in the hydrogen molecule. To count sigma and pi bonds, draw the lewis dot structure and count the single, double and triple bonds present. And a hybridized orbital cannot be involved in a pi bond. * all the atoms have linear geometry. Various bond parameters such as bond length, bond angle, and bond enthalpy depend on the way the overlapping of atomic orbital takes place.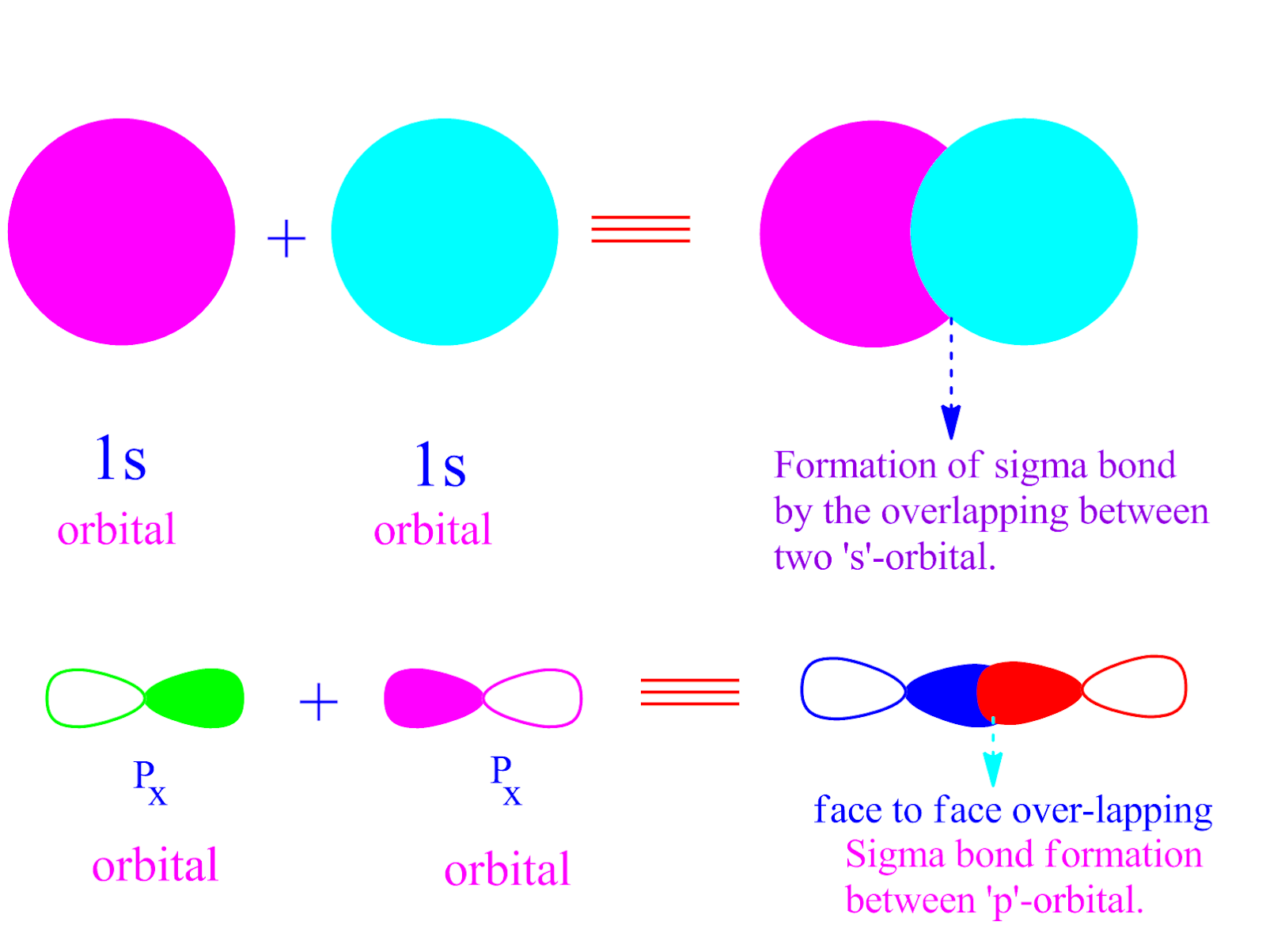
Why are sigma bond more stronger than pi bond ? PG.CHEMEASY
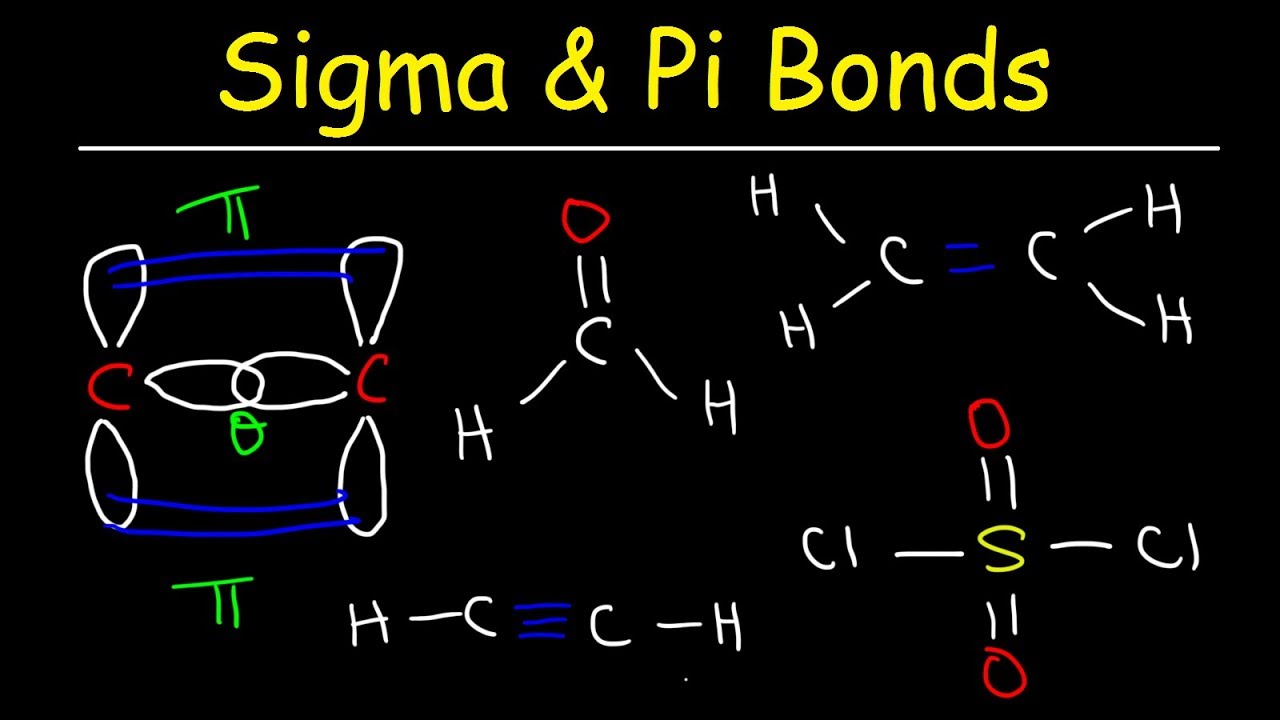
Sigma and Pi Bonds Explained, Basic Introduction, Chemistry Inflation
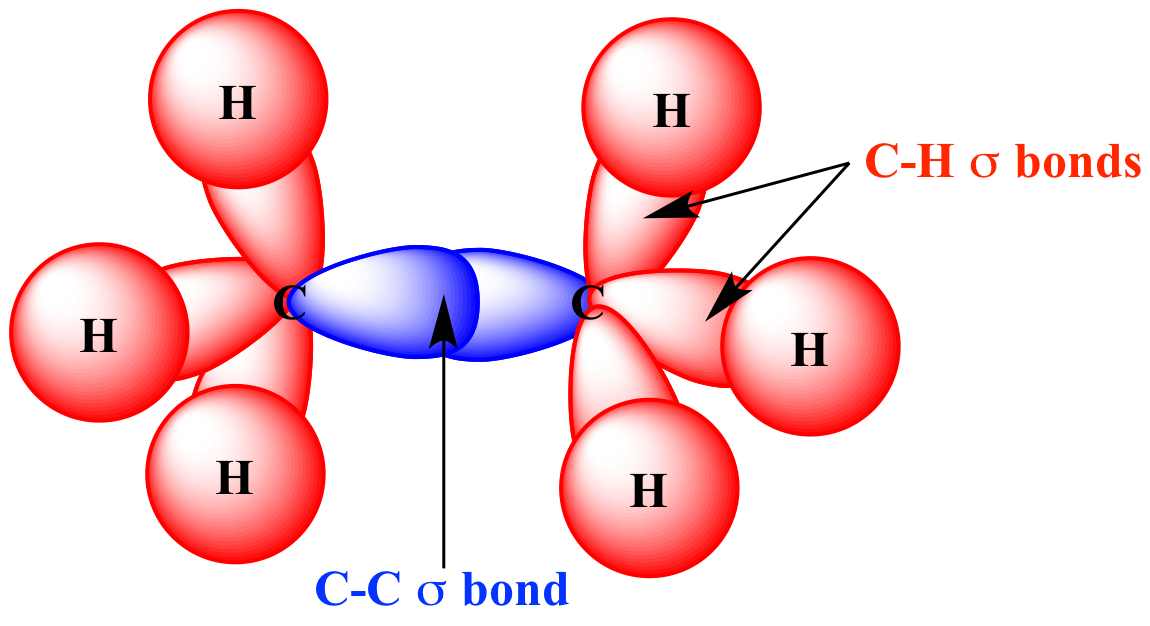
Illustrated Glossary of Organic Chemistry Sigma bond (σ bond)
Sigma Bonding Is Most Simply Defined For Diatomic Molecules Using The Language And Tools Of.
A Sigma Bond Is The First Bond Between Two Atoms, And Is The Strongest Sort Of Covalent Bond.
9.4 Sigma And Pi Bonds | General Chemistry.
As Illustrations, Consider The Bonds That Have Already Been Studied.
Related Post: