How To Draw The Electron Configuration
How To Draw The Electron Configuration - Under the orbital approximation, we let each electron occupy an orbital, which can be solved by a single wavefunction. The alkali metal sodium (atomic number 11) has one more electron than the neon atom. Web the easiest way to create electron configurations is using an electron configuration table, which is a way of writing down the various orbitals available to electrons. What is an electron configuration? 1.1m views 9 years ago chemistry: Electrons must occupy the lowest available shell, closest to the nucleus. Web an electron configuration diagram is a model that depicts the position of electrons as they orbit the nucleus of an atom. Shell the maximum number of electrons that can fill each is: Web electron configurations give us insight into the bonds that atoms are likely to form and the relative stability of ions. For example, the electron configuration of sodium is 1s 2 2s 2 2p 6 3s 1. Here are a few highlights that you learned in the last section about bohr's model: This video also includes ques. Identify and explain exceptions to predicted electron configurations for atoms and ions. How to draw and write electronic structures (also called the electron configurations).an essential. It explains how to write the orbital diagram n. A lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. Identify and explain exceptions to predicted electron configurations for atoms and ions. The alkali metal sodium (atomic number 11) has one more electron than the neon atom. For example, the electron configuration of sodium is 1s 2 2s 2 2p 6 3s 1. Shared. We're talking about an s orbital in the first energy level, so we could label this orbital as being the one s orbital. 133k views 9 years ago. The first shell has the lowest energy and the energies increase as the electrons get further away from the positively charged nucleus. Web when writing an electron configuration, first write the energy. Web use these steps to draw electron configuration diagrams for the first 20 elements in the periodic table. Web the easiest way to create electron configurations is using an electron configuration table, which is a way of writing down the various orbitals available to electrons. Electrons must occupy the lowest available shell, closest to the nucleus. Web this video will go through how to draw an atoms electron structure (electronic configuration), there are 2 methods you can use. The first shell has the lowest energy and the energies increase as the electrons get further away from the positively charged nucleus. Web when writing an electron configuration, first write the energy level (the period), then the subshell to be filled and the superscript, which is the number of electrons in that subshell. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. Shells are the allowed energy levels of electrons. We're talking about an s orbital in the first energy level, so we could label this orbital as being the one s orbital. Web chemistry 1.1 (gmit) 5: Web electron configurations give us insight into the bonds that atoms are likely to form and the relative stability of ions. A simple tutorial on how to draw electron. Web electron configurations describe where electrons are located around the nucleus of an atom. Electron configuration diagrams | properties of matter | chemistry | fuseschool learn the basics about drawing electron. 77 views 8 months ago c1 atomic structure. The rules above allow one to write the electron configurations for all the elements in the periodic table.
Drawing electron configuration diagrams Chemistry for All The Fuse
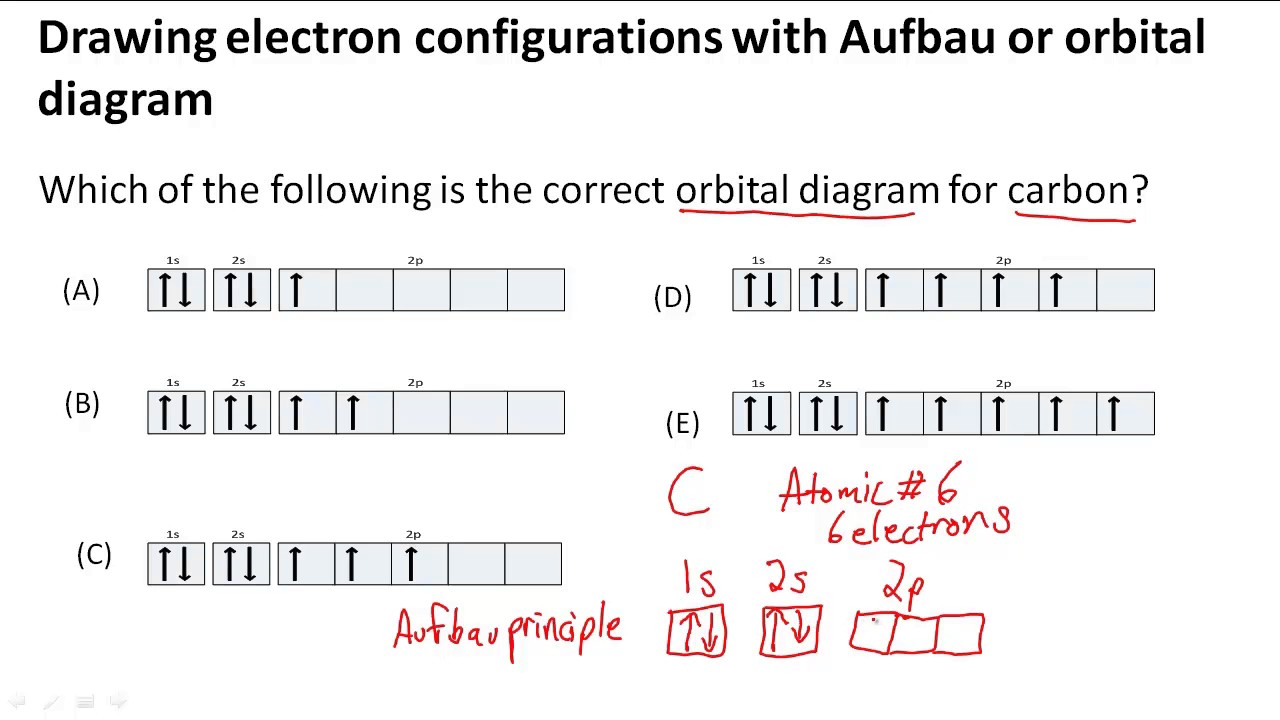
Drawing electron configurations with Aufbau/orbital diagram YouTube
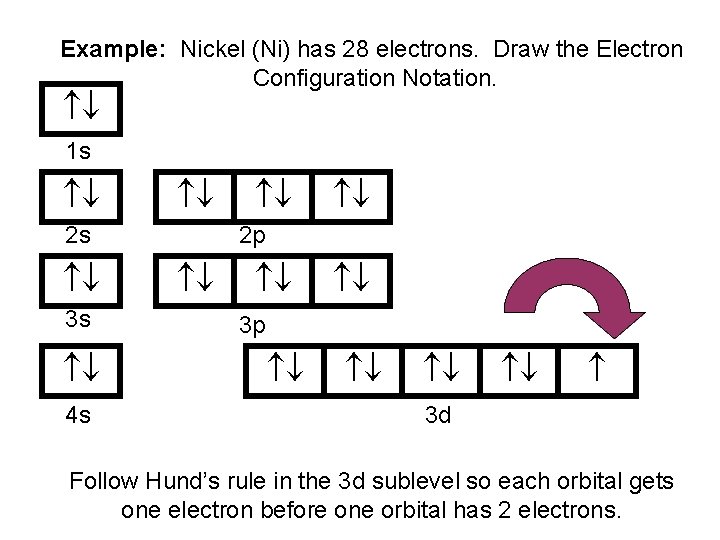
Electron Configuration Chapter 5 Electrons have 3 levels
Here Are A Few Highlights That You Learned In The Last Section About Bohr's Model:
The Electron Configuration Is The Standard Notation Used To Describe The Electronic Structure Of An Atom.
Identify And Explain Exceptions To Predicted Electron Configurations For Atoms And Ions.
Shell The Maximum Number Of Electrons That Can Fill Each Is:
Related Post: