How To Draw The Conjugate Base Of An Acid
How To Draw The Conjugate Base Of An Acid - Calculate the k for the conjugate base of chlorous acid. A weak base partially dissociates into hydroxide ions and its conjugate acid at equilibrium. Conjugate acid and conjugate base. 856k views 7 years ago new ap & general chemistry video playlist. For the following named acids: (b) ch 3 nh 2; If acetic acid donates this proton, then the electrons in magenta are left behind on the oxygen, so the conjugate base would have a. So h₂co₃ is the conjugate acid of hco₃⁻. Web what is the conjugate acid or the conjugate base of (a) hcl; Chemistry, inorganic, organic, physical, physical chemistry. Web let's draw the conjugate base for acetic acid. A weak base partially dissociates into hydroxide ions and its conjugate acid at equilibrium. A conjugate acid is formed when a proton is added to a base, and a. Calculate the k for the conjugate base of chlorous acid. Conjugate acid and conjugate base. Calculate the k for the conjugate base of chlorous acid. Chemistry, inorganic, organic, physical, physical chemistry. A conjugate acid is formed when a proton is added to a base, and a. Explain conjugate acids of bases. So this is the conjugate acid on the right. Calculate the pka for chlorous acid. So this is the conjugate acid on the right. Web identify the lewis acid, lewis base, the conjugate acid and the conjugate base in the reaction above. The pka value for [fe(h 2 o) 6] 2+ is 9.5; Consider the reaction ah (+) + h₂o →> a: Calculate the k for the conjugate base of chlorous acid. So this is the conjugate acid on the right. (0) in chemistry class, your professor talks about conjugate acid. 856k views 7 years ago new ap & general chemistry video playlist. Chemistry, inorganic, organic, physical, physical chemistry. This chemistry video explains the concept of acids. Web ha + h2o ⇌ ha + h 2 o ⇌ h3o+ + a− h 3 o + + a − in which ha acts as acid. Web let's draw the conjugate base for acetic acid. Chlorous acid (hclo) has a k of 0.011. Calculate the pka for chlorous acid. (b) ch 3 nh 2; A weak base partially dissociates into hydroxide ions and its conjugate acid at equilibrium. Web identify the lewis acid, lewis base, the conjugate acid and the conjugate base in the reaction above. The pka value for [fe(h 2 o) 6] 2+ is 9.5; For the following named acids: This problem has been solved!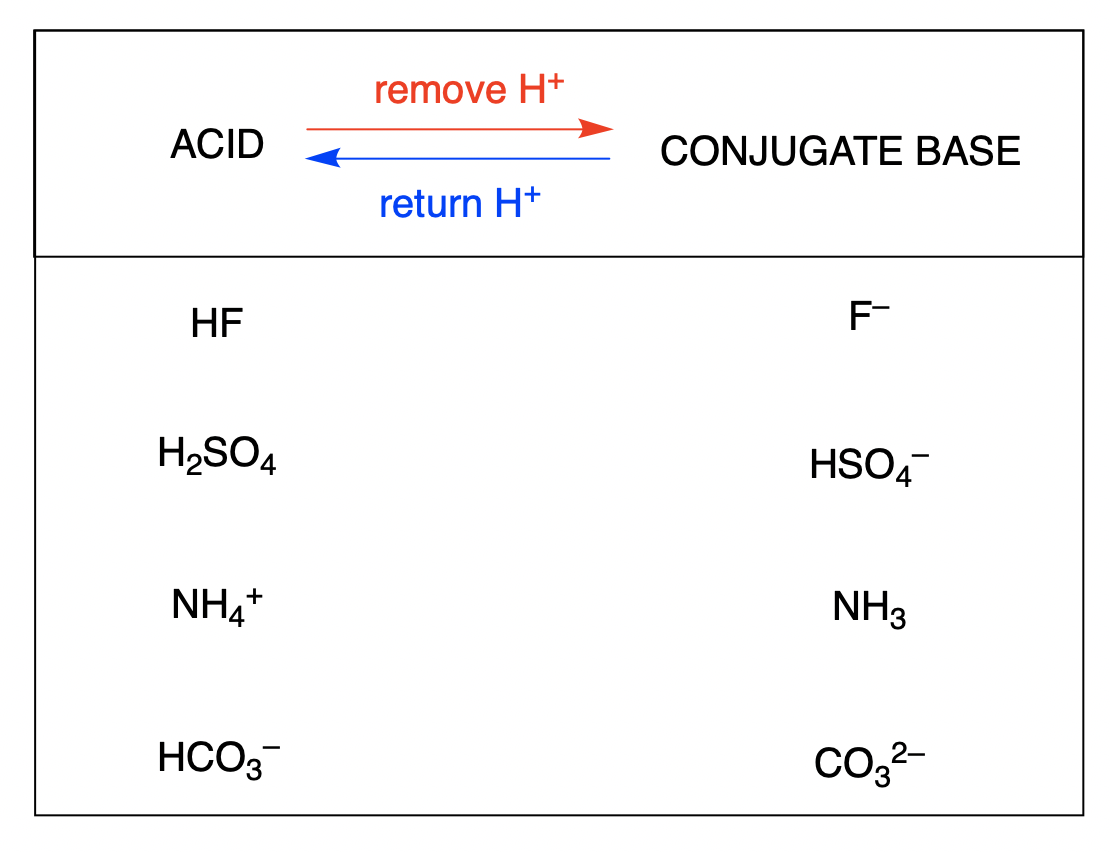
5.1 AcidBase Definitions & Conjugate AcidBase Pairs General

PPT Acids and Bases PowerPoint Presentation, free download ID1919310

Acids and Bases (Alevel) ChemistryStudent
A Conjugate Acid Is Formed When A Proton Is Added To A Base, And A.
Web So On The Right, This Right Here Must Be The Conjugate Acid.
We See That Hco₃⁻ Becomes H₂Co₃.
If Acetic Acid Donates This Proton, Then The Electrons In Magenta Are Left Behind On The Oxygen, So The Conjugate Base Would Have A.
Related Post: