How To Draw Molecular Orbital Diagram
How To Draw Molecular Orbital Diagram - In the first column, draw the atomic diagram for your first element. Web this video discusses how to draw the molecular orbital (mo) diagram for the b2 (+) molecule. General chemistry supplement (eames) molecular orbital theory. Web how to draw molecular orbital diagrams for conjugated systems. Web molecular orbital diagrams are complex, involving two additional orbitals, electronegativity, atomic symmetries and atomic energies. How to draw a molecular orbital diagram. Determine the total number of valence electrons in the he 2 2 + ion. With oxygen, you know that the atomic orbital potential energies go in the following order: Web the molecular orbital theory allows one to predict the distribution of electrons in a molecule which in turn can help predict molecular properties such as shape, magnetism, and bond order. Answer • count the valence electrons on the molecule. That's the number of valence electrons on. Web the objective of this wiki is to provide readers with the fundamental steps in constructing simple homonuclear and heteronuclear diatomic molecular orbital diagrams. Web how might one draw atomic and molecular orbital diagrams? Web this chemistry video tutorial provides a basic introduction into molecular orbital theory. It describes the formation of bonding. From this diagram, calculate the bond order for [latex]\ce{o2}[/latex]. V 1s << v 2s < v 2p. How does this diagram account for the paramagnetism of [latex]\ce{o2}[/latex]? They also demonstrate the molecule’s bond order, or how many bonds are shared between the two atoms. Construct a molecular orbital diagram of the kind shown in this lesson for a simple diatomic. Valence bond theory is able to explain many aspects of bonding, but not all. Web drawing molecular orbital diagrams is one of the trickier concepts in chemistry. From this diagram, calculate the bond order for [latex]\ce{o2}[/latex]. To complement this theory, we use another called the molecular orbital (mo) theory. Define bond order, and state its significance. How does this diagram account for the paramagnetism of o 2? Answer • count the valence electrons on the molecule. Valence bond theory is able to explain many aspects of bonding, but not all. Molecular orbital theory is a more sophisticated model for understanding the nature of chemical bonding. Draw two lines to create three columns. From this diagram, calculate the bond order for o 2. Web the objective of this wiki is to provide readers with the fundamental steps in constructing simple homonuclear and heteronuclear diatomic molecular orbital diagrams. That's the number of valence electrons on. General chemistry supplement (eames) molecular orbital theory. The bond order of the boron cation is also calculated and the meaning of this number is. It describes the formation of bonding and antibonding molecular orbitals from the combination of. How do you populate the electrons? Web describe the essential difference between a sigma and a pi molecular orbital. To complement this theory, we use another called the molecular orbital (mo) theory. Web molecular orbital diagrams are complex, involving two additional orbitals, electronegativity, atomic symmetries and atomic energies. These steps may then be extrapolated to construct more difficult polyatomic diagrams.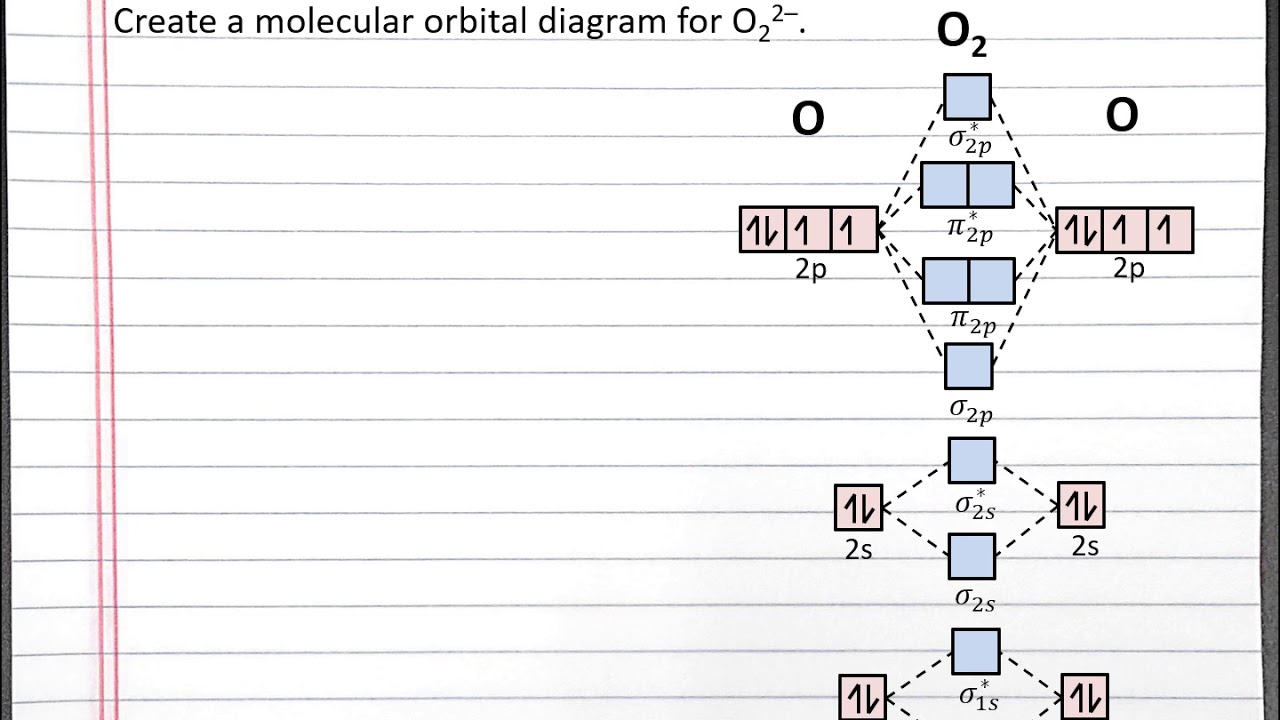
CHEM 101 Creating a Molecular Orbital Diagram for a Diatomic Ion in
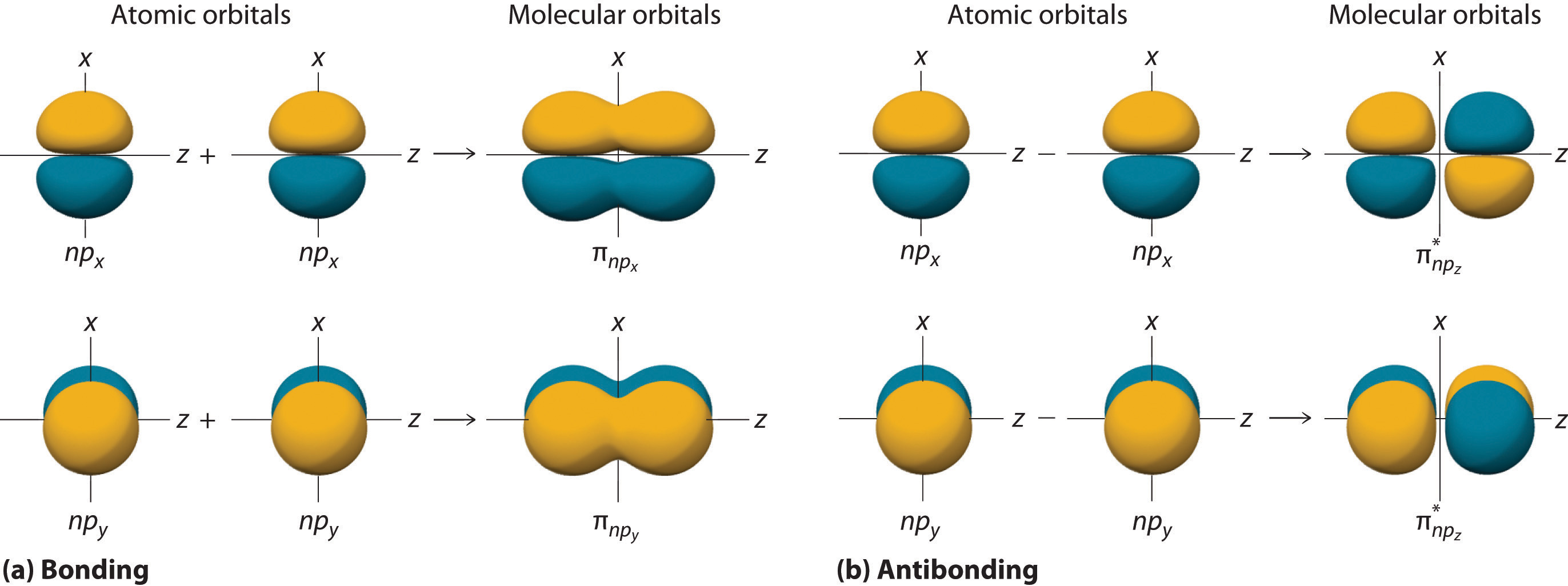
10.5 Molecular Orbital Theory Chemistry LibreTexts

how to draw molecular orbital diagram for heteronuclear molecules
The First Major Step Is Understanding The Difference Between Two Major Theories:
Web To Understand The Bonding Of A Diatomic Molecule, A Molecular Orbital Diagram Is Used.
From This Diagram, Calculate The Bond Order For [Latex]\Ce{O2}[/Latex].
In The First Column, Draw The Atomic Diagram For Your First Element.
Related Post: