How To Draw Lewis Dot Structures For Ionic Compounds
How To Draw Lewis Dot Structures For Ionic Compounds - Web how can i draw lewis dot structures for ionic compounds? Note down a skeletal structure displaying a realistic bonding pattern by means of only the element. 292k views 3 years ago new ap & general chemistry video playlist. Web draw lewis structures for ionic compounds. In section 4.7, we demonstrated that ions are formed by losing electrons to make cations, or by gaining electrons to form anions. Web draw lewis structures for ionic compounds. Web to use the lewis structure calculator follow these steps: The two ions attract each other according to coulombic interactions. Lewis structure for ionic compounds. A lewis electron dot formula comprises one dot for every valence electron and an element’s symbol. Examples for drawing lewis structures for covalent bonds. Covalent bonds work because electrons are shared. When atoms have fewer than eight electrons, they tend to react and form more stable compounds. How to draw lewis dot structure? Web draw lewis structures depicting the bonding in simple molecules. Stages to articulate the electron dot formula are stated beneath. Web students will draw lewis dot structures for various compounds and show how ions are formed through the transfer of electrons. Chemistry covalent bonds drawing lewis structures. Atoms & elements 4h 15m. Using the periodic table to draw lewis dot structures. 224k views 5 years ago. Web how do you draw the lewis structure for ionic compounds? Note down a skeletal structure displaying a realistic bonding pattern by means of only the element. What is a lewis dot structure? Lewis structure for ionic compounds. Remember that lewis dot structures. How to draw a lewis structure. The example is for the nitrate ion. A lewis structure is a diagram that shows the chemical bonds between atoms in a molecule and the valence electrons or lone pairs of electrons. Web the steps to draw the lewis dot structure of ionic compounds are as follows: In all cases, these bonds involve the sharing or transfer of. Intro to general chemistry 3h 51m. Chad explains and demonstrates exactly how to draw lewis. A lewis electron dot formula comprises one dot for every valence electron and an element’s symbol. Stages to articulate the electron dot formula are stated beneath. The two ions attract each other according to coulombic interactions. Note down a skeletal structure displaying a realistic bonding pattern by means of only the element. 8.2 how to draw lewis dot structures | complete guide | general chemistry. Draw lewis dot structures for ionically bonded compounds. When discussing the octet rule, we do not consider d or f. In an ionic bond, one atom looses all its outer electrons (leaving behind a filled inner shell) while another atom gains electron (s) to fill its valence shell.
Formation of Ionic Compounds using Dot Structures YouTube
![[DIAGRAM] Ionic Bond Drawing Lewis Dot Diagrams](https://image.slideserve.com/334634/lewis-dot-structure-of-clo-4-by-bonds-table-l.jpg)
[DIAGRAM] Ionic Bond Drawing Lewis Dot Diagrams
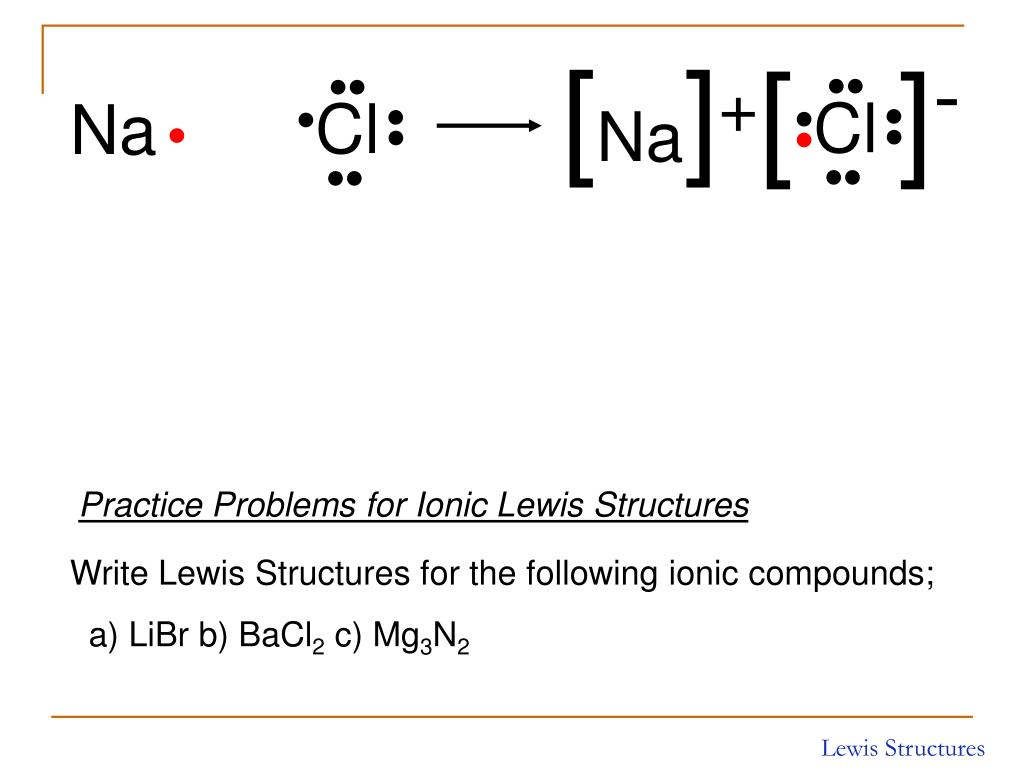
Lewis Structure Of Ionic Compounds
How To Draw Electron Dot Structures?
How To Draw Lewis Dot Structures.
Web Draw Lewis Structures Depicting The Bonding In Simple Molecules.
3.2 Lewis Structure Of An Anion.
Related Post: