How To Draw Electron Orbitals
How To Draw Electron Orbitals - In this case, sulfur has 16 electrons that need to be placed into orbitals. This table is easy to remember, and it makes it possible to generate the electron configuration table for any given element. Atomic orbitals come in different shapes, depending on how much energy and angular momentum is associated with that orbital. This is known as hund's rule. It looks something like this. Web for example, the 2p shell has three p orbitals. Elements are placed in order on the periodic table based on their atomic number, how many protons they have. Web we're going to look at what orbitals are, what they represent, how electrons go in orbitals, the order electrons go in orbitals, and the shapes of orbitals. Web the easiest way to create electron configurations is using an electron configuration table, which is a way of writing down the various orbitals available to electrons. Although such drawings show the relative sizes of the orbitals, they do not normally show the spherical nodes in the 2 s and 3 s orbitals because the. The aufbau principle, the pau. Electron configurations describe where electrons are located around the nucleus of an atom. Orbital diagrams are a visual way to show where the electrons are located within an atom. In this case, sulfur has 16 electrons that need to be placed into orbitals. For example, the electron configuration of lithium, 1s²2s¹, tells us that lithium. 1s 2 2s 2 2p 1. Lithium, containing three electrons, has two electrons occupying an s orbital at the first energy level, and one electron occupying an s orbital at the second energy level. The aufbau principle, the pau. Web electron orbitals are mathematical functions that describe the probability of finding an electron around the nucleus of an atom. Orbital. Each picture is domain coloring of a ψ (x, y, z) function which depends on the coordinates of one electron. Orbital diagrams must follow 3 rules: Web the filling order follows: For example, the electron configuration of lithium, 1s²2s¹, tells us that lithium has two electrons in the 1s subshell and one electron in the 2s subshell. Orbital diagrams are. Elements are placed in order on the periodic table based on their atomic number, how many protons they have. The aufbau principle, the pau. It looks something like this. Web the electrons in an atom are arranged in shells that surround the nucleus, with each successive shell being farther from the nucleus. Web electrons are represented by dots or crosses and are positioned in energy levels, or ‘shells’, around the central nucleus. Web the easiest way to create electron configurations is using an electron configuration table, which is a way of writing down the various orbitals available to electrons. Web the specific arrangement of electrons in orbitals of an atom determines many of the chemical properties of that atom. To draw orbitals, always start at the lowest energy level and build up. Electron configuration of nitrogen and oxygen atoms You will also learn how to use hund'. 1s, 2s, 2p x, 2p y, and 2p z. Each picture is domain coloring of a ψ (x, y, z) function which depends on the coordinates of one electron. Web this video goes over how to properly draw orbital diagrams for an element, after determining the electron configuration. This is sometimes called the bohr, or the ‘solar system’, model. In this case, sulfur has 16 electrons that need to be placed into orbitals. For example, the electron configuration of lithium, 1s²2s¹, tells us that lithium has two electrons in the 1s subshell and one electron in the 2s subshell.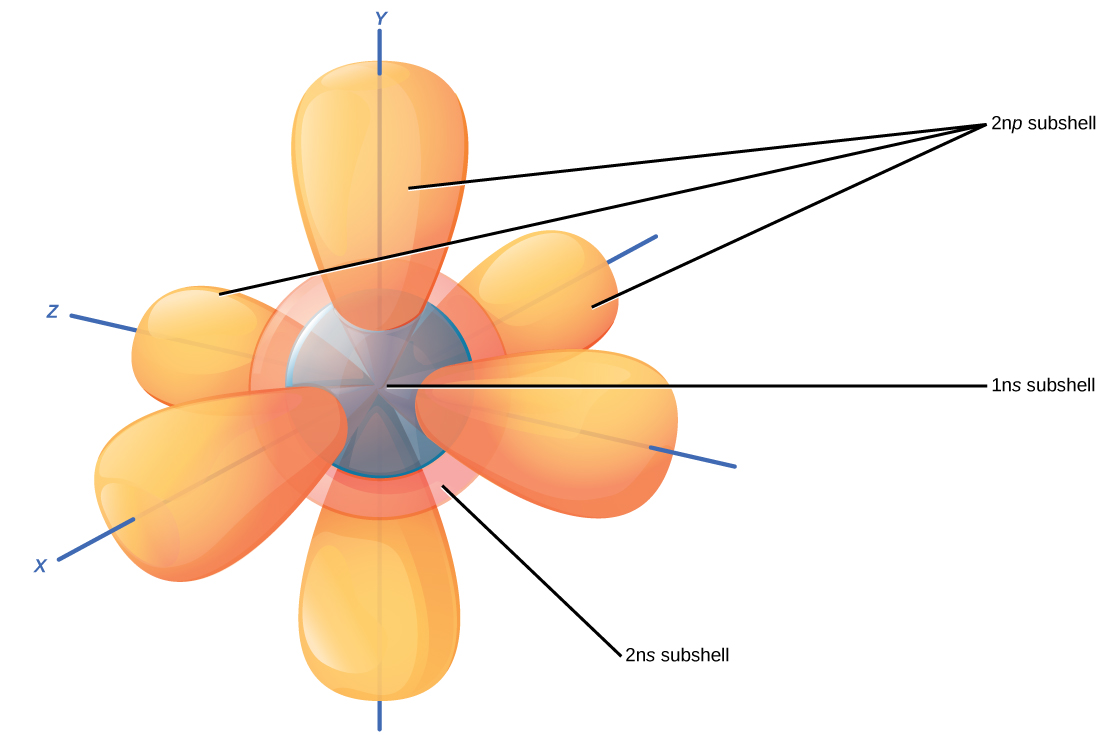
Electrons Biology for Majors I

How to Draw Shapes of Orbitals

Electron Orbitals (ALevel) ChemistryStudent
This Is Known As Hund's Rule.
This Table Is Easy To Remember, And It Makes It Possible To Generate The Electron Configuration Table For Any Given Element.
Remember, Arrows Represent Electrons, And Lines Or Boxes Are The Orbitals.
A 1S Orbital Holding 2 Electrons Would Be Drawn As Shown On The Right, But It Can Be Written Even More Quickly As 1S 2.
Related Post: