How To Draw Dipole Moments
How To Draw Dipole Moments - For one bond, the bond dipole moment is determined by the difference in electronegativity between the two atoms. ∣∣ p→∣∣ ≡ q d (1.4.1) (1.4.1) | p → | ≡ q d. Identify the direction of all dipole moments. Molecular geometry and dipole moments. Web this organic chemistry video tutorial provides a basic introduction into dipole moment and molecular polarity. Mathematically, dipole moment (µ) = charge (q) * distance of separation (r) Web using the equation above, the dipole moment is calculated to be 1.85 d by multiplying the distance between the oxygen and hydrogen atoms by the charge difference between them and then finding the components of each that point in the direction of the net dipole moment (the angle of the molecule is 104.5˚). The net dipole is the measurable, which is called the dipole. The difference in electronegativity can be used to. Web to calculate the dipole moment of a chemical bond, the following formula is used: Steps for determining molecular dipole moments: Molecular geometry and dipole moments. Web using the equation above, the dipole moment is calculated to be 1.85 d by multiplying the distance between the oxygen and hydrogen atoms by the charge difference between them and then finding the components of each that point in the direction of the net dipole moment (the angle. To define electronegativity and bond polarity. Web this chemistry video tutorial provides a basic introduction into bond polarity, electronegativity, and the dipole moment of a bond. Looking at the electronegativity and shape of the h2o molecule tells you how the arrow depicts the polarity: Web this organic chemistry video tutorial provides a basic introduction into dipole moment and molecular polarity.. To calculate the percent ionic character of a covalent polar bond. Learn about carbon dioxide's dipole moment, see how bond dipoles work and understand what molecular. 1 debye is equal to. The direction of the dipole moment is that it points from the negative charge to the positive charge. Web it is relatively easy to measure dipole moments: Web it is relatively easy to measure dipole moments: Write the lewis structure of the molecule or polyatomic ion. Web now that we understand how to draw dot structures and we know how to predict the shapes of molecules, let's use those skills to analyze the polarity of molecules, using what's called the dipole moment. You must be able to combine your knowledge of molecular shapes and bond polarities to determine whether or not a given compound will have a dipole moment. Explain how dipole moments depend on both molecular shape and bond polarity. 1 debye is equal to. The dipole moments of molecules. From in between the hydrogen atoms to the oxygen atom. Web this chemistry video tutorial provides a basic introduction into bond polarity, electronegativity, and the dipole moment of a bond. Predict whether a molecule will possess a dipole moment, given only its molecular formula or kekulé structure. Mathematically, dipole moment (µ) = charge (q) * distance of separation (r) The dipole moment is the measure of the polarity of a molecule. Show the net dipole moment as the vector sum of the individual dipole moments. I will teach you the super easy trick to. ∣∣ p→∣∣ ≡ q d (1.4.1) (1.4.1) | p → | ≡ q d. A dipole moment is the product of the magnitude of the charge and the distance between the centers of the positive and negative charges.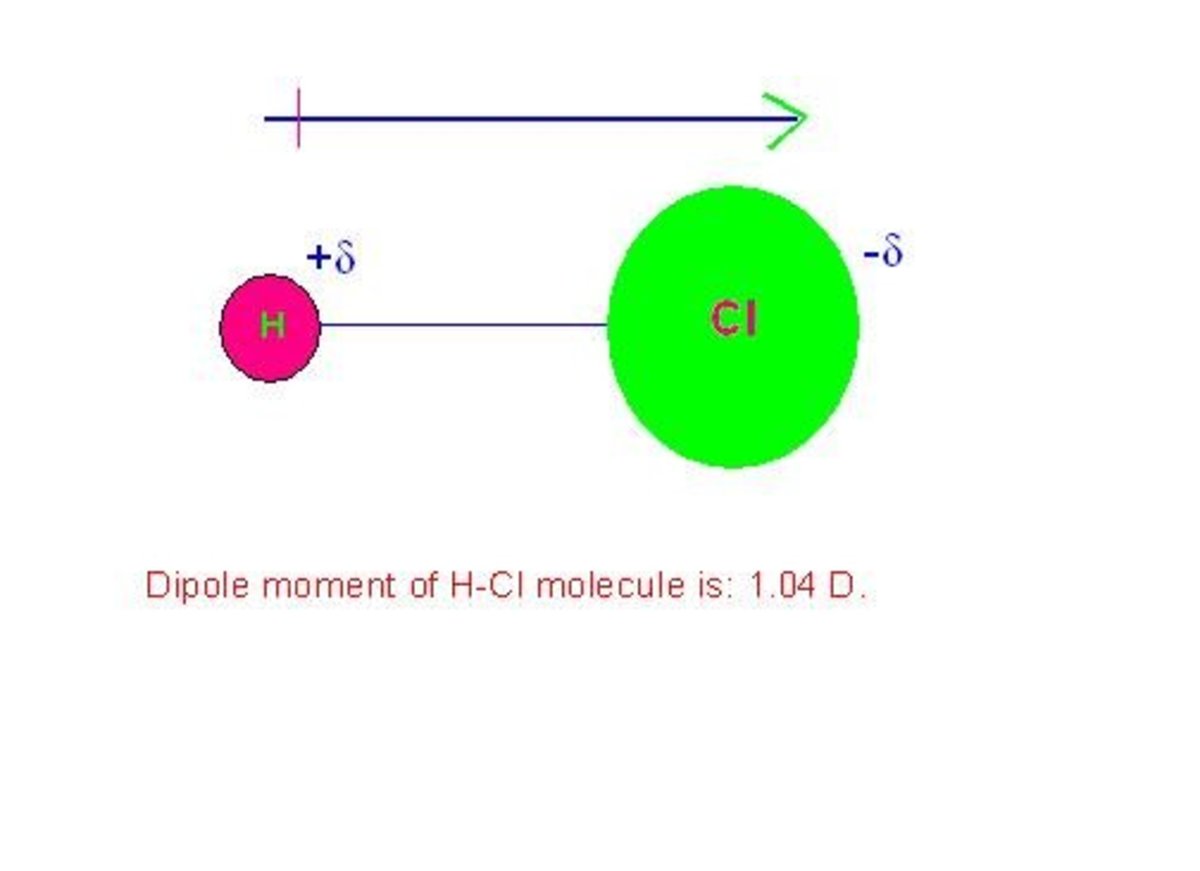
Lucid Understanding Of, “Dipole Moment Of A Molecule” HubPages
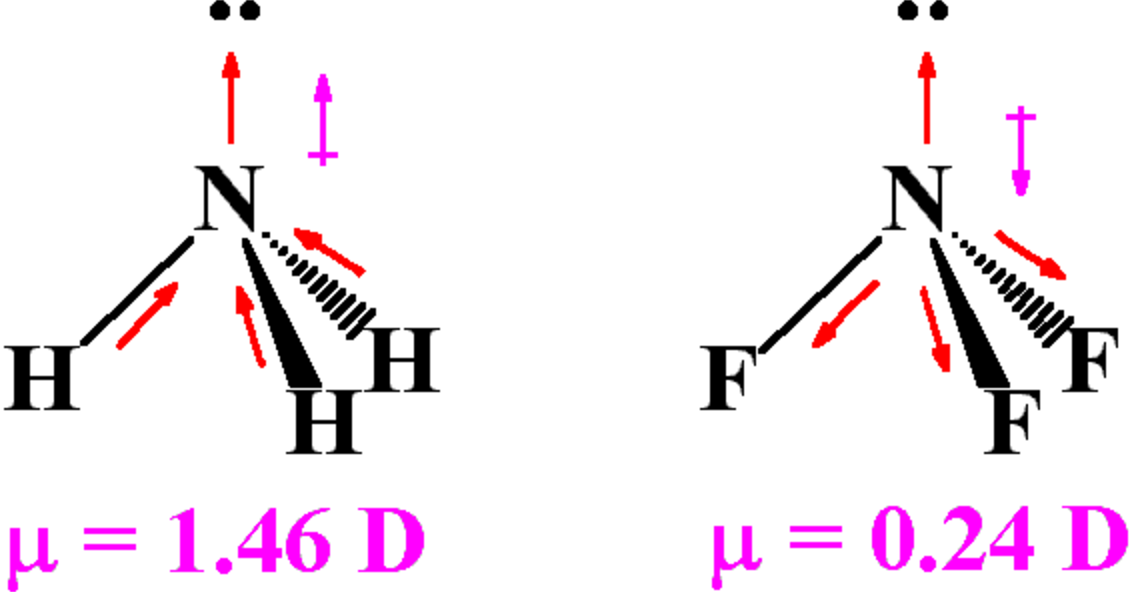
Dipole Moments Chemical Bonding and Molecular Structure, Chemistry

Bond Polarity, Electronegativity and Dipole Moment Chemistry Practice
This Lecture Is About Dipole Moment In Chemistry.
The Vsepr Theory Can Be Used To Determine The Electron Pair Geometries And Molecular Structures As Follows:
9, Part 1) Jared Christensen.
Using The Cross Bow Arrow Shown Below We Can Show That It Has A Net Dipole.
Related Post: