How To Draw Bohr Models
How To Draw Bohr Models - 2 electrons can go in the first shell, 8 in the second, 8 in the third, and so on. Draw the electrons in their respective orbitals. Draw the first electron shell and put the electrons as a dot in it. The protons and neutrons are placed into the nucleus and the. • the electronic configuration of silicon is 1s22s22p63s23p2. Silicon (si) 2, 8, 4: Drawing a shell model diagram and an energy diagram for hydrogen, and then using the diagrams to calculate the energy required to excite an electron between different energy levels. How to draw a bohr model. The number of shells that you draw will depend on the number of electrons in the atom. Web drawing bohr model of silicon. Write the number of protons and neutrons at the center of the nucleus. Sodium (na) 2, 8, 1: Web drawing bohr models 3. Web a bohr model of a chlorine atom shows a nucleus surrounded by three concentric rings. Draw the first shell 4. Draw the nucleus of an atom. Draw a circle to represent the nucleus of the atom. Aluminum (al) 2, 8, 3: Web drawing bohr model of silicon. 2 electrons can go in the first shell, 8 in the second, 8 in the third, and so on. The first three shells should contain 2, 8, and 18 electrons, respectively, while the fourth shell holds the remaining 1 electron. Draw the first electron shell and put the electrons as a dot in it. Two in the first shell, eight in the second shell, eight in the third shell. Home school chemistry day 15 unit 2: Aluminum (al) 2,. Draw the nucleus of an atom. 31k views 8 years ago. The protons and neutrons are placed into the nucleus and the. • the atomic number of silicon is 14. Home school chemistry day 15 unit 2: Web now, to draw the bohr model for boron we first need to identify the number of different atomic particles that this atom contains. The first three shells should contain 2, 8, and 18 electrons, respectively, while the fourth shell holds the remaining 1 electron. Write the number of protons and neutrons at the center of the nucleus. E ( n) = − 1 n 2 ⋅ 13.6 ev. Web discuss how the bohr model can be used to explain atomic spectra. The highest energy form of electromagnetic waves are gamma (γ) rays and the lowest energy form are radio. Add your electrons, the 2nd shell can hold up to 8 be sure to add them one at a time Drawing a shell model diagram and an energy diagram for hydrogen, and then using the diagrams to calculate the energy required to excite an electron between different energy levels. 4.5k views 2 years ago atomic structure. Bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus. The facts that can be derived from it are: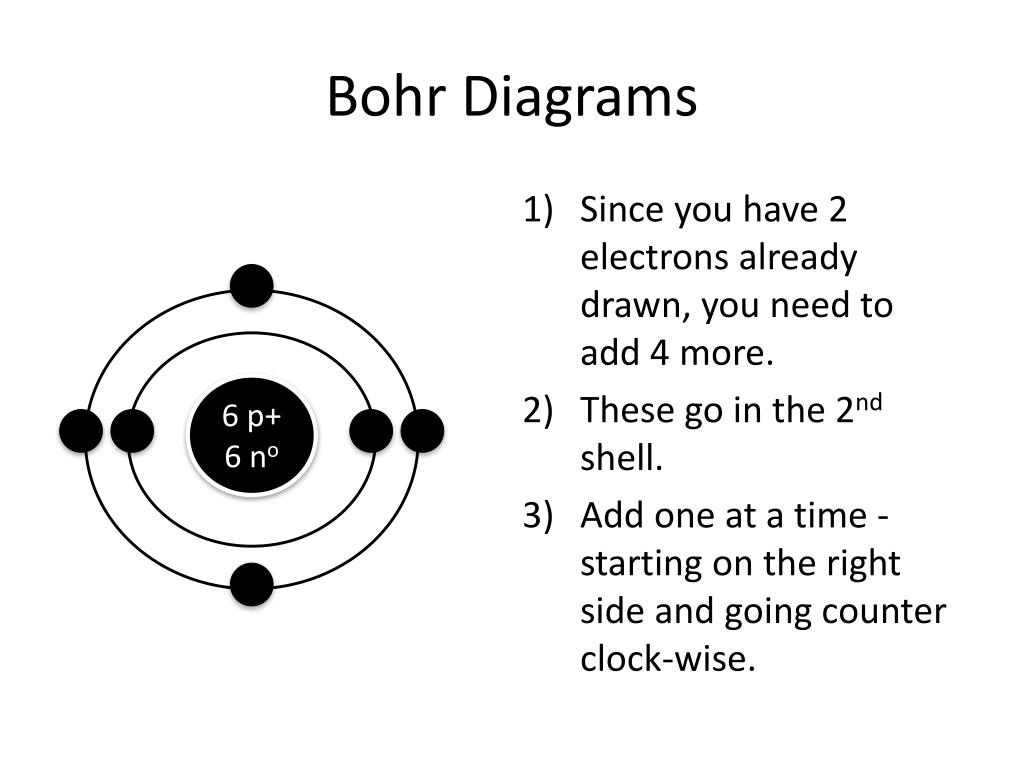
PPT How to Draw Bohr Diagrams PowerPoint Presentation, free download

How to draw a Bohr model of an atom BohrRutherford Diagrams
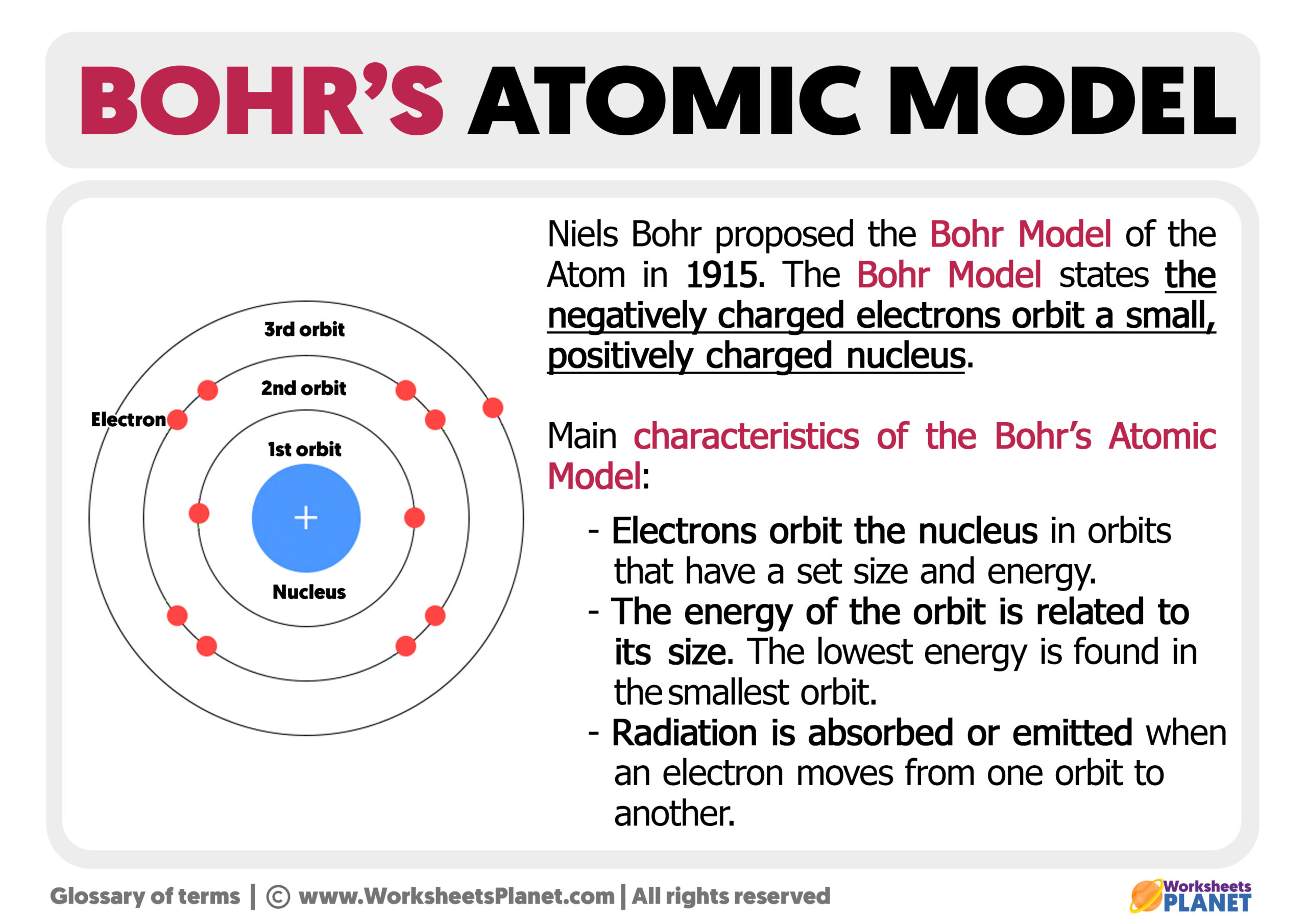
Bohr's Atomic Model
Sodium (Na) 2, 8, 1:
Web Drawing Bohr Models 3.
This Lesson Will Walk Your Students Through The Basics On How To Draw A Bohr Diagram For The First 20 Elements On The Periodic Table.
• The Electronic Configuration Of Silicon Is 1S22S22P63S23P2.
Related Post: