Electrons Drawing
Electrons Drawing - Web a lewis electron dot symbol (or electron dot diagram or a lewis diagram or a lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. Draw lewis structures for covalent compounds. The example is for the nitrate ion. Web build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. For example, the electron configuration of lithium, 1s²2s¹, tells us that lithium has two electrons in the 1s subshell and one electron in the 2s subshell. It's easier to understand electron configuration and valence if you can actually see the electrons surrounding atoms. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. The remaining two electrons occupy the 2p subshell. Take sodium as an example. The following procedure can be used to construct lewis electron structures for more complex molecules and ions: Then play a game to test your ideas! Electrons must occupy the lowest available shell, closest to the nucleus. This indicates not only that they are electrons, but helps remind the viewer that electrons contain a negative charge. The remaining two electrons occupy the 2p subshell. Use these steps to draw electron configuration diagrams for the first 20 elements in. 8 electrons in the second shell. In order for an electron to be in the electron cloud of an atom, it must be in. For example, the electron configuration of lithium, 1s²2s¹, tells us that lithium has two electrons in the 1s subshell and one electron in the 2s subshell. Web the best way to draw electrons is to draw. (hydrogen is excluded because it can hold a. For example, the electron configuration of lithium, 1s²2s¹, tells us that lithium has two electrons in the 1s subshell and one electron in the 2s subshell. Web updated on november 05, 2019. The example is for the nitrate ion. Draw lewis structures for covalent compounds. Use these steps to draw electron configuration diagrams for the first 20 elements in the periodic table. So let's say we wanted to draw the dot structure for this molecule, so silicon tetrafluoride. Web specifically, an element’s position in the periodic table helps you figure out its electron configuration, how the electrons are organized around the nucleus. Web updated on november 05, 2019. For example, the electron configuration of lithium, 1s²2s¹, tells us that lithium has two electrons in the 1s subshell and one electron in the 2s subshell. The remaining two electrons occupy the 2p subshell. It's easier to understand electron configuration and valence if you can actually see the electrons surrounding atoms. The example is for the nitrate ion. Carbon (atomic number 6) has six electrons. Take sodium as an example. 8 electrons in the second shell. Web when drawing orbital diagrams, we include empty boxes to depict any empty orbitals in the same subshell that we are filling. Web in this section, we will explore the typical method for depicting valence shell electrons and chemical bonds, namely lewis symbols and lewis structures. 2 electrons in the first shell. (hydrogen is excluded because it can hold a. Want to join the conversation?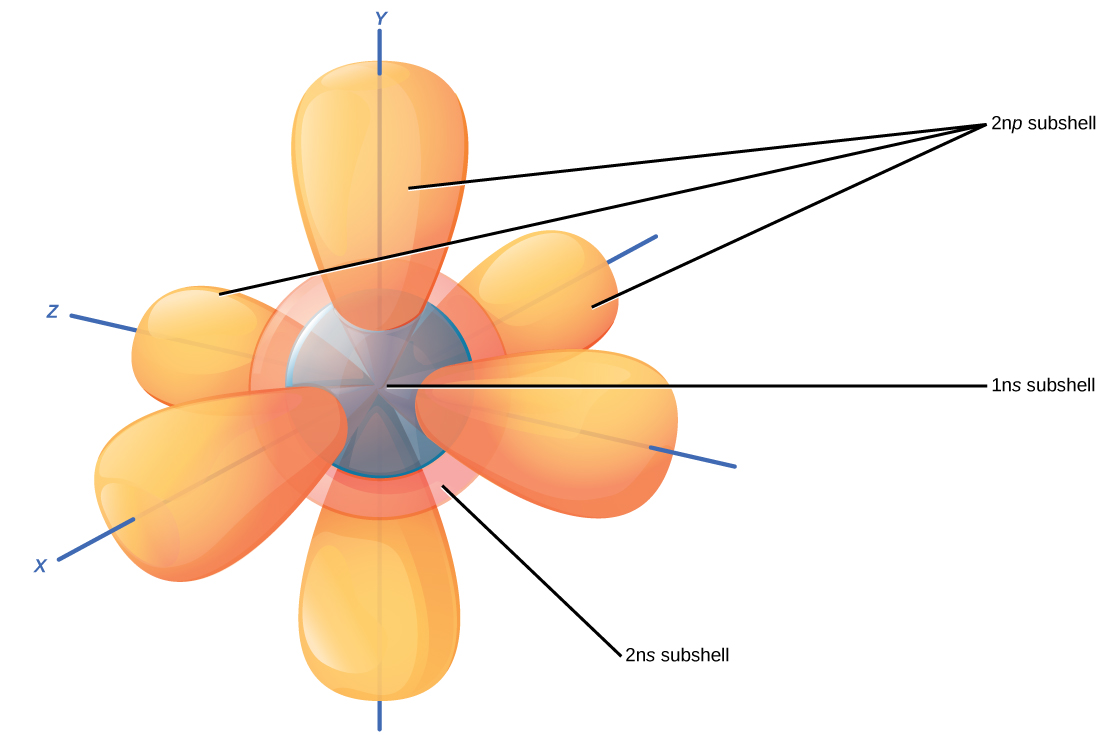
Electrons Biology for Majors I

Atomic Structure Broad Learnings
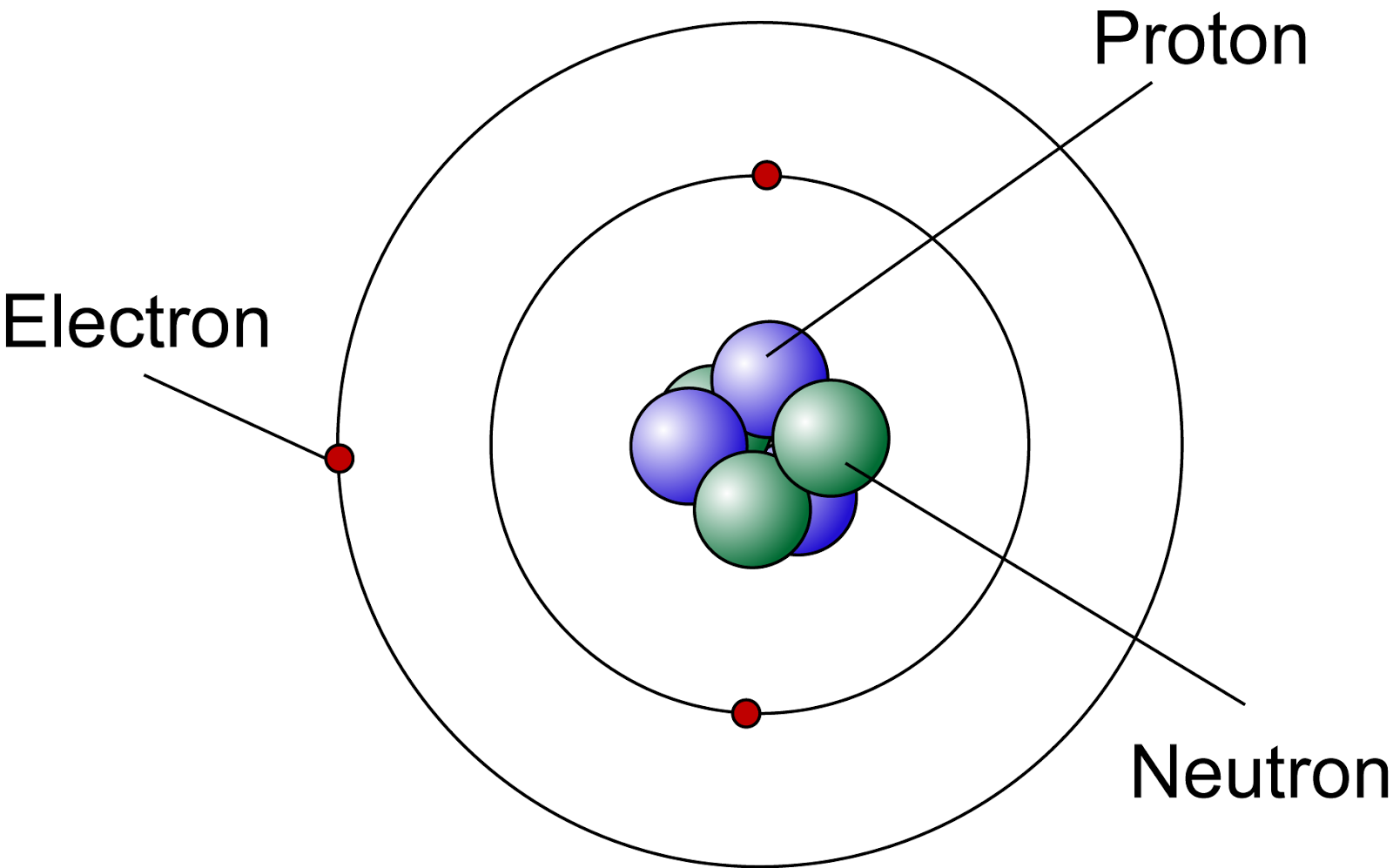
Lets Get Inside An Atom!! The Science Station
Web Writing And Drawing Electronic Configuration.
Web Construct An Atom According To The Bohr Model.
A Rule Stating That Atoms Lose, Gain, Or Share Electrons In Order To Have A Full Valence Shell Of 8 Electrons.
The First Thing We Would Need To Do Is To Find The Total Number Of Valence Electrons.
Related Post: