Electron Drawings
Electron Drawings - For example, the electron configuration of lithium, 1s²2s¹, tells us that lithium has two electrons in the 1s subshell and one electron in the 2s subshell. Every element on the periodic table consists of atoms, which are composed of protons, neutrons, and electrons. Begin by drawing a circle. Examples of how to draw electron. An orbital is the quantum mechanical refinement of bohr’s orbit. Before assigning the electrons of an atom into orbitals, one must become familiar with the basic concepts of electron configurations. Rules for electron orbital charts. Draw an incomplete oval around the nucleus. Web explore molecule shapes by building molecules in 3d! Diagrams contain a lot of useful information in a compact format. Web the electron configurations and orbital diagrams of these four elements are: So if you wanted to write down the electron configuration for beryllium (4 electrons), you’d start at the top and pass through 1s, and then you’d loop around until you reached 2s. Use the link below to answer the following questions: Since the core electron shells correspond to. In all cases, these bonds involve the sharing or transfer. What is an electron orbital diagram? We construct the periodic table by following the aufbau principle (from german, meaning “building up”). How does molecule shape change with different numbers of bonds and electron pairs? This forms the first electron. For example, the electron configuration of lithium, 1s²2s¹, tells us that lithium has two electrons in the 1s subshell and one electron in the 2s subshell. Electrons are represented by dots or crosses and are positioned in energy levels, or ‘shells’, around the central nucleus. Also included is a downloadable version of this atom drawing lesson. Electron configuration chart of. The dot diagrams are the same for each element in the representative element groups. In all cases, these bonds involve the sharing or transfer. Thus far in this chapter, we have discussed the various types of bonds that form between atoms and/or ions. Electrons are represented by dots or crosses and are positioned in energy levels, or ‘shells’, around the central nucleus. The alkali metal sodium (atomic number 11) has one more electron than the neon atom. Electron dot diagrams show the valence electrons for an atom. The number of dots equals. This forms the first electron. An orbital is the quantum mechanical refinement of bohr’s orbit. You can learn how to draw an atom by following along with this engaging drawing guide. Electrons are represented by dots or crosses and are positioned in energy levels, or ‘shells’, around the central nucleus. How to draw orbital diagrams. First we determine the number of electrons in the atom; The football play diagrammed above describes the lineup of each player on the team and describes how they will move when the ball is snapped. How does molecule shape change with different numbers of bonds and electron pairs? Use the link below to answer the following questions: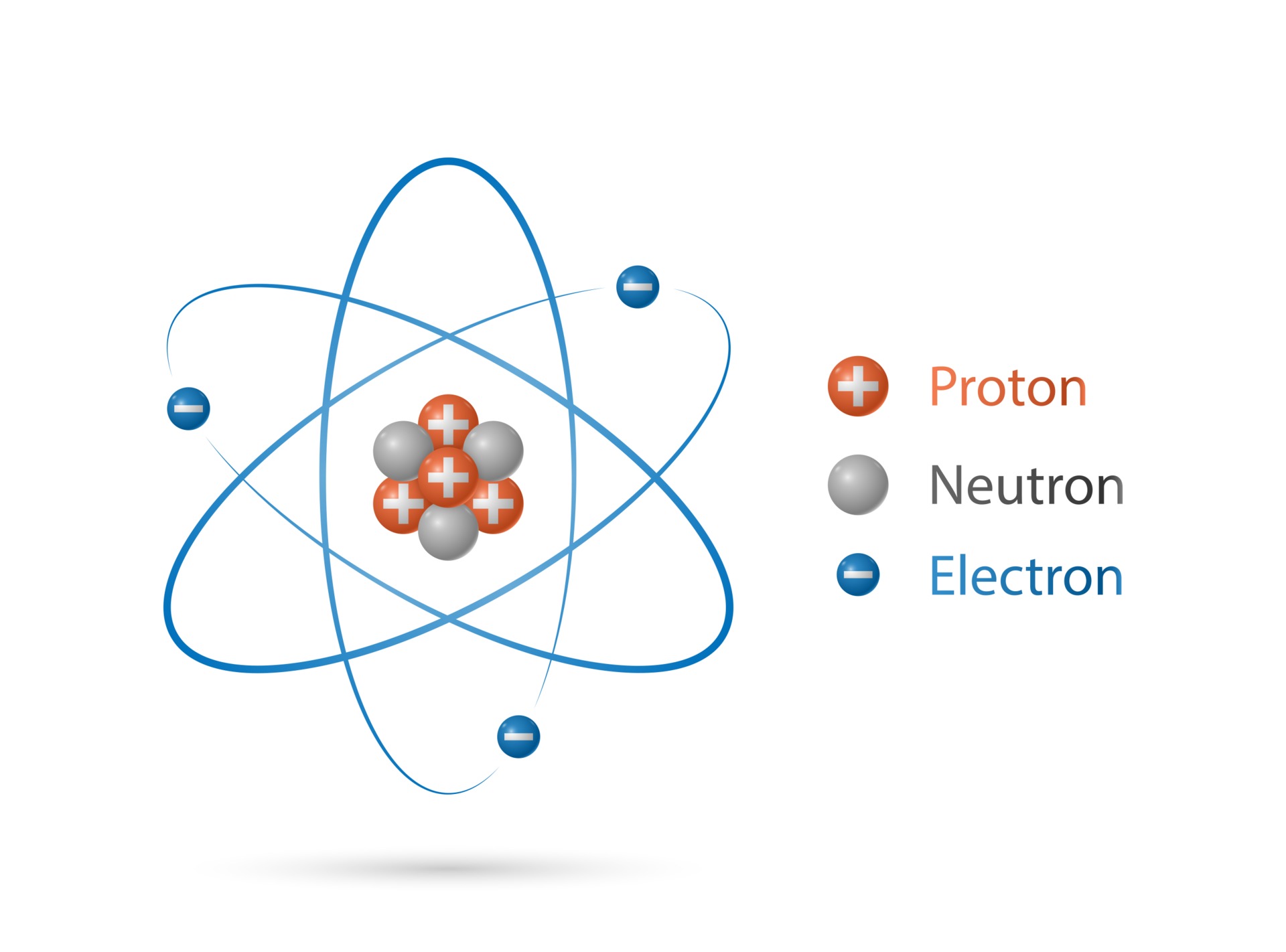
modelo de estructura de átomo, núcleo de protones y neutrones
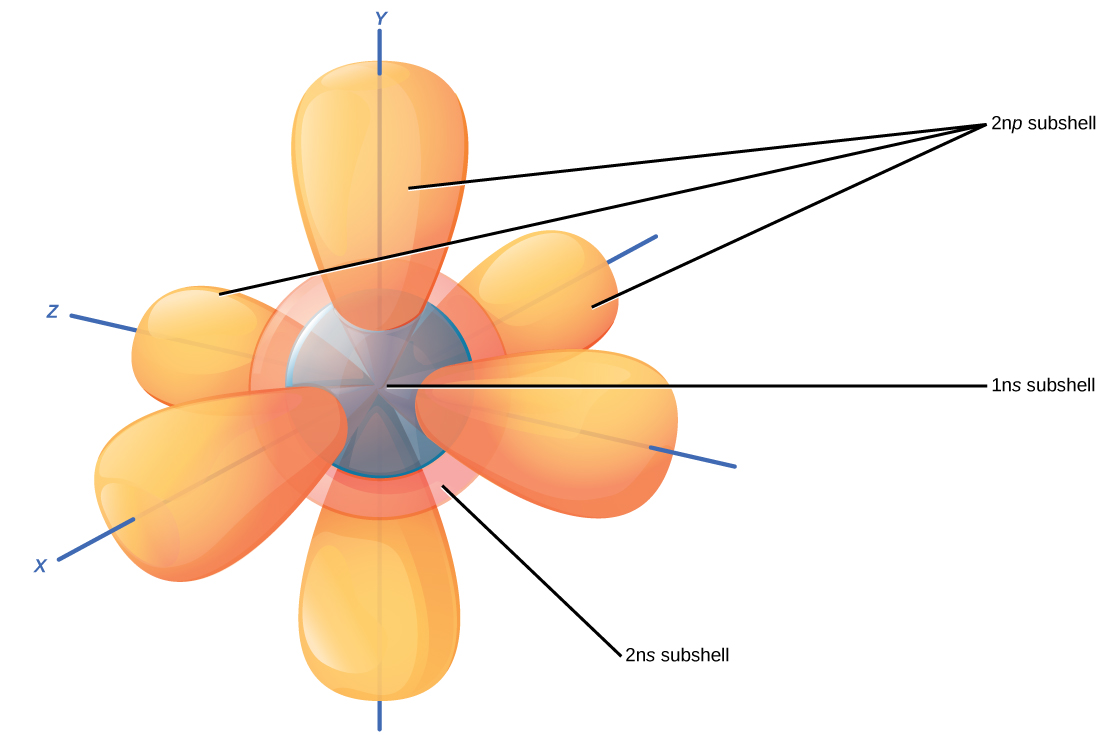
Electrons Biology for Majors I

3.7 Electron Arrangement The Quantum Model Chemistry LibreTexts
The Shorthand Electron Configuration (Or Noble Gas Configuration) As Well As Full Electron Configuration Is Also Mentioned In The Table.
A Lewis Diagram Shows How The Valence Electrons Are Distributed Around The Atoms In A Molecule.
Then, Compare The Model To Real Molecules!
Shared Pairs Of Electrons Are Drawn As Lines Between Atoms, While Lone Pairs Of Electrons Are Drawn As Dots Next To Atoms.
Related Post: