Drawing Transition States
Drawing Transition States - Eyring, evans, and polyani have clarified for us that we’re looking for a saddle point on the potential energy surface. Their existence and their structure must be inferred from other information, especially that which pertains the surrounding states. Web draw an energy diagram for the following s n 2 reaction. Web draw reaction energy diagrams from the thermodynamic and kinetic data/information; Use a reaction energy diagram to discuss transition states, ea, intermediates & rate determining step; Therefore, transition states cannot be physically or experimentally observed, much less studied. Web in order to gain some insight into what a particular transition state looks like, chemists often invoke the hammond postulate, which states that a transition state resembles the structure of the nearest stable species. Unlike minima, one of the second derivatives in the first order saddle is negative. Here is a picture of a likely transition state for a substitution reaction between hydroxide and chloromethane: Like minima, the first order saddle points are stationary points with all forces zero. The transition state) closest to the species highest in energy. Web learn how to draw a state transition diagram using various techniques and tools. In this webcast, we develop a general procedure for thinking about and drawing transition states based on a given. A transition state has partial bonds, an extremely short lifetime (measured in femtoseconds), and cannot be isolated.. Strictly speaking, a transition state of a chemical reaction is a first order saddle point. Web learn how to draw a state transition diagram using various techniques and tools. Web transition states correspond to saddle points on the potential energy surface. Like minima, the first order saddle points are stationary points with all forces zero. 2k views 10 years ago. 15k views 5 years ago. This video explains how to draw a state transition diagram by state transition table. Create clear and concise diagrams to visualize the flow of. They are the middle state between reactants and products. Web transition states are when there are the dashed lines indicating broken or formed bonds. Web transition state theory was proposed in 1935 by henry erying, and further developed by merrideth g. Understand the process of representing the behavior of a system through states, events, and transitions. For an exergonic reaction, therefore, the transition state resembles the reactants more than it does the products. Web in order to gain some insight into what a particular transition state looks like, chemists often invoke the hammond postulate, which states that a transition state resembles the structure of the nearest stable species. Web either way it must go down to a more stable state. 9.5k views 12 years ago. Therefore, transition states cannot be physically or experimentally observed, much less studied. Strictly speaking, a transition state of a chemical reaction is a first order saddle point. A minimax point) is a point on a function where the derivative in all directions is zero. Label the axes, the ea, the δ h ° and the transition state of the reaction. Unlike minima, one of the second derivatives in the first order saddle is negative. Draw the transition state of a reaction Web learn how to draw a state transition diagram using various techniques and tools. Web transition states are when there are the dashed lines indicating broken or formed bonds. Their existence and their structure must be inferred from other information, especially that which pertains the surrounding states. Web the energy difference between reactants and the transition state is called the activation energy, δg ‡, and determines how rapidly the reaction occurs at a given temperature.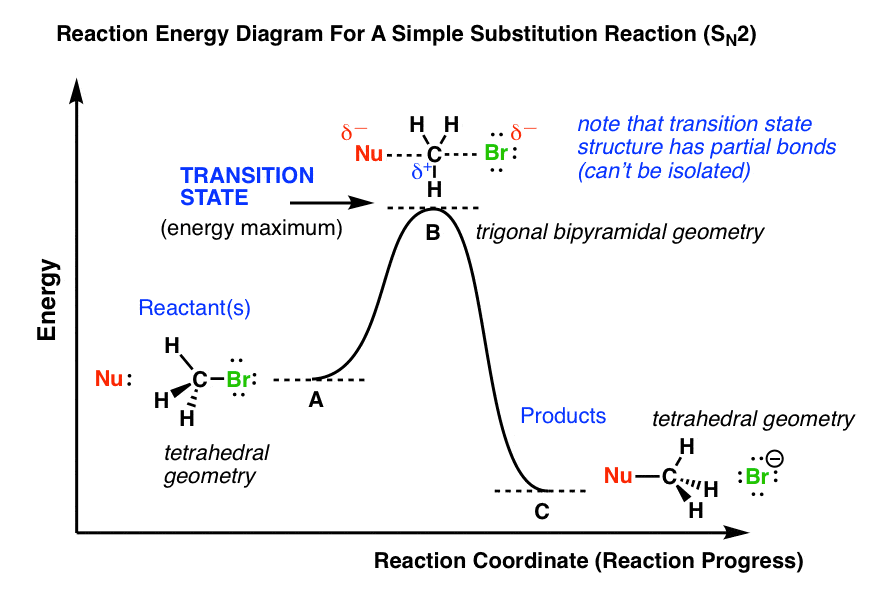
5 membered transition state

What is the Difference Between a Transition State and an Intermediate

01.02 Transition States Revisited YouTube
Given A Sigmatropic Rearrangement Starting Material, Accurately Predict The Product Including Stereochemistry.
A Transition State Has Partial Bonds, An Extremely Short Lifetime (Measured In Femtoseconds), And Cannot Be Isolated.
So If The Slow Step Is The First Step The Graph Will Have A Higher Hump On The First Transition Step.
Draw The Structure Of Reactants And Products On The Diagram.
Related Post: