Drawing Orbital Diagrams
Drawing Orbital Diagrams - Web this video goes over how to properly draw orbital diagrams for an element, after determining the electron configuration. Intro to general chemistry 3h 51m. Each box represents one orbital, and each arrow indicates one electron. A p orbital along the y axis is labeled p y and one along the z axis is a p z orbital. Atoms & elements 4h 15m. Typically, they only show the outermost electrons. For example, the electron configuration of lithium, 1s²2s¹, tells us that lithium has two electrons in the 1s subshell and one electron in the 2s subshell. Web learn how to draw orbital diagrams. Want to join the conversation? The remaining two electrons occupy the 2p subshell. It explains how to write the orbital diagram n. Web a p orbital which extends along the x axis is labeled a p x orbital. Start by drawing a large circle in the center of your paper. The remaining two electrons occupy the 2p. Carbon (atomic number 6) has six electrons. Web commonly, the electron configuration is used to describe the orbitals of an atom in its ground state, but it can also be used to represent an atom that has ionized into a cation or anion by compensating with the loss of or gain of electrons in their subsequent orbitals. Web an orbital box diagram can be written as well.. Web how to draw orbital diagrams. The remaining two electrons occupy the 2p. An orbital is a space where a specific pair of electrons can be found. The orbital box diagrams are listed for the first 20 elements in the figure below. To use molecular orbital theory to predict bond order. Start by drawing a large circle in the center of your paper. Web steps for drawing an orbital diagram. The arrow shows a qualitative representation of increasing orbital energy. Four of them fill the 1s and 2s orbitals. Write out the electron configuration to determine which orbitals are filled. Web the bohr model shows the atom as a central nucleus containing protons and neutrons, with the electrons in circular electron shells at specific distances from the nucleus, similar to planets orbiting around the sun. Get a pencil (preferably a mechanical pencil for precision), a good eraser, a ruler, and a sheet of drawing paper. In contrast to his concept of a simple circular orbit with a fixed radius, orbitals are mathematically derived regions of space with different probabilities of containing an electron. Web an orbital box diagram can be written as well. For example, the orbital diagram of li can be shown as: Each box represents one orbital, and each arrow indicates one electron. To use molecular orbital theory to predict bond order. Web learn how to draw orbital diagrams. Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. For example, the electron configuration of lithium, 1s²2s¹, tells us that lithium has two electrons in the 1s subshell and one electron in the 2s subshell. Want to join the conversation?
how to draw molecular orbital diagram for heteronuclear molecules
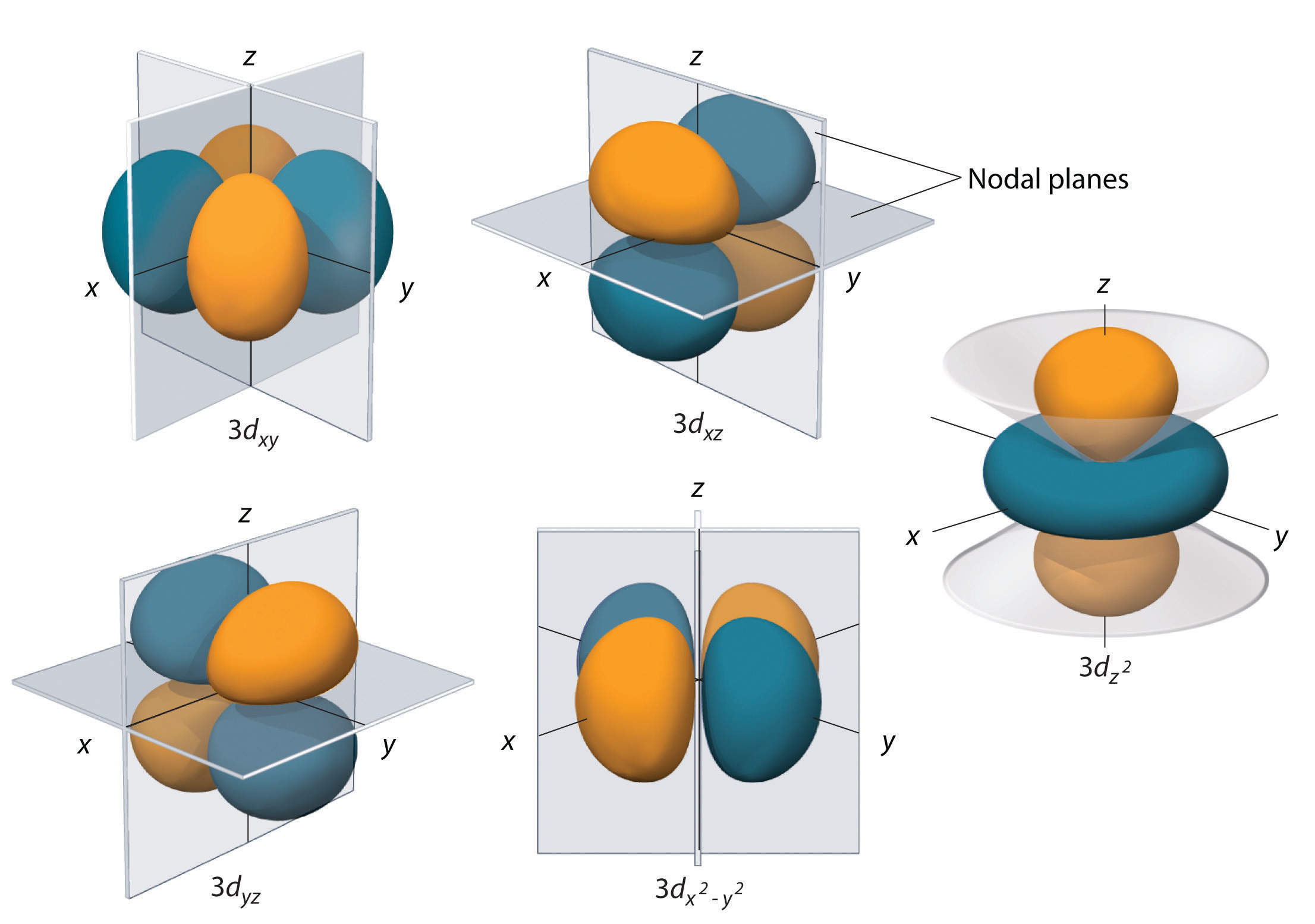
6.6 3D Representation of Orbitals Chemistry LibreTexts
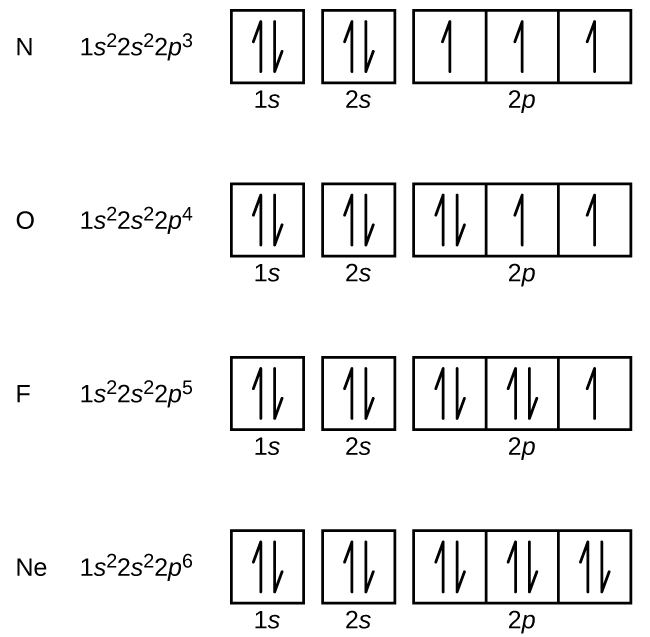
Electron Configurations, Orbital Box Notation (M7Q7) UWMadison
Four Of Them Fill The 1S And 2S Orbitals.
The Remaining Two Electrons Occupy The 2P.
We Classified The Different Orbital Into Shells And Sub Shells To Distinguish Them More Easily.
Draw A Long Vertical Arrow That Points Upward.
Related Post: