Drawing Of Water Molecule
Drawing Of Water Molecule - Web the configuration of the water molecule. It is one of the easiest atoms to build a model of, and is therefore an excellent starting point for students learning to build molecular models. Web understanding the structure and characteristics of water molecules is essential for comprehending these processes. Once you’ve drawn a molecule, you can click the 2d to 3d button to convert the molecule into a 3d model which is then. In the covalent bond between oxygen and hydrogen, the oxygen atom attracts electrons a bit more strongly than the hydrogen atoms. Identify three special properties of water that make it unusual for a molecule of its size, and explain how these result from hydrogen bonding. Solid (ice), liquid (water), and gas (steam). The bent shape of the water molecule is critical because the polar o−h o − h bonds do not cancel one another and the molecule as a whole is polar. The water molecule, as a whole, has 10 protons and 10 electrons, so it is neutral. A water molecule consists of two hydrogen atoms bonded to an oxygen atom, and its overall structure is bent. It is well known that water consists of two elements, hydrogen (h) and oxygen (o). This guide will provide a comprehensive approach to drawing water molecules, allowing you to visually represent this vital molecule and gain insights into its unique properties. Number of total valence electrons of oxygen and hydrogen atoms are used to draw lewis structure. Web with 70%. Web lewis structure of water molecule contains two single bonds around oxygen atom. 4.9k views 2 years ago chemistry learning videos. Web choose from water molecule drawing stock illustrations from istock. 2.3), which constitutes the water molecule (h 2 o). This is because the oxygen atom, in addition to forming bonds with the hydrogen atoms, also carries two pairs of. There are 3 different forms of water, or h 2 o: Once you’ve drawn a molecule, you can click the 2d to 3d button to convert the molecule into a 3d model which is then. This guide will provide a comprehensive approach to drawing water molecules, allowing you to visually represent this vital molecule and gain insights into its unique. It is well known that water consists of two elements, hydrogen (h) and oxygen (o). This guide will provide a comprehensive approach to drawing water molecules, allowing you to visually represent this vital molecule and gain insights into its unique properties. Students will be able to explain, on the molecular level, what makes water a polar molecule. This molecule also has another chemical name of dihydrogen monoxide. Solid (ice), liquid (water), and gas (steam). There are two lone pairs of electrons on each oxygen atom (represented. Number of total valence electrons of oxygen and hydrogen atoms are used to draw lewis structure. The bent shape of the water molecule is critical because the polar o−h o − h bonds do not cancel one another and the molecule as a whole is polar. Web this drawing highlights two h 2 o molecules, one at the surface, and the other in the bulk of the liquid. In a water molecule, the oxygen atom and hydrogen atoms share electrons in covalent bonds, but the sharing is not equal. Web choose from water molecule drawing stock illustrations from istock. The water molecule, visualized three different ways: Web lewis structure of water molecule contains two single bonds around oxygen atom. We will walk through the steps below to construct the molecular orbital diagram of water. Explain what is meant by hydrogen bonding and the molecular structural features that bring it about. The water molecule, as a whole, has 10 protons and 10 electrons, so it is neutral.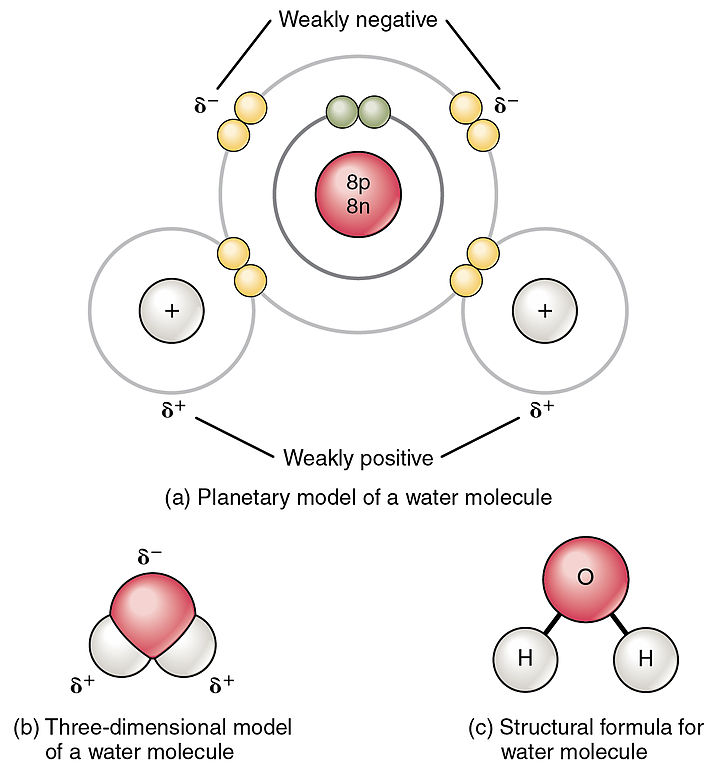
Water Principles of Biology
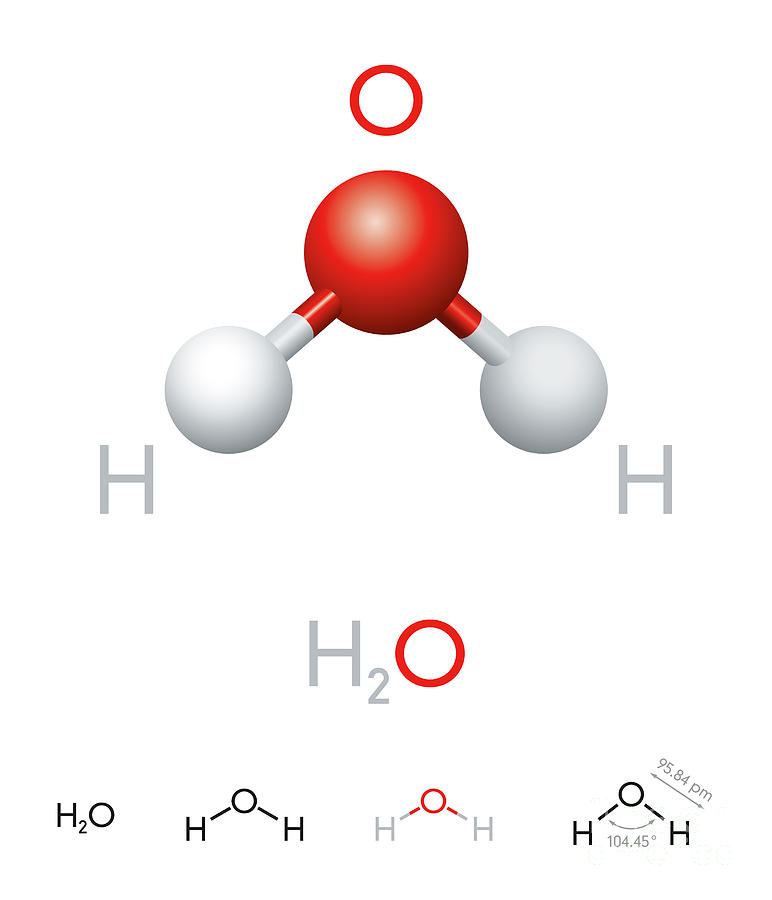
H2O Water molecule model and chemical formula Digital Art by Peter
Water molecule vector drawing Public domain vectors
It Is One Of The Easiest Atoms To Build A Model Of, And Is Therefore An Excellent Starting Point For Students Learning To Build Molecular Models.
Web The Configuration Of The Water Molecule.
Each Step Of Drawing Lewis Structure Of H 2 O Are.
It Is A Simple Molecule, Consisting Of Just One Oxygen Atom And Two Hydrogen Atoms.
Related Post: