Drawing Of Chlorine
Drawing Of Chlorine - The nature bond and place of atoms in a molecule is determined by the lewis dot structure of that molecule, the electronic configuration of chlorine is as follows, Web construct a qualitative molecular orbital diagram for chlorine, cl 2. Web steps of drawing lewis structure of ccl 4. Compare the bond order to that seen in the lewis structure (remember that an electron in an antibonding orbital cancels the stabilization due to bonding of an electron in a bonding orbital). The atomic number of this chemical element is 17. There are several steps to complete the lewis structure of ccl 4. Web how to draw lewis structure for cl 2. Web learn about the bohr diagram of chlorine and how to draw it. Write protons, neutrons, and electrons of chlorine atom. Draw nucleus of chlorine atom. Web how to draw lewis structure for cl 2. Web steps of drawing lewis structure of ccl 4. Web to illustrate the chlorine orbital diagram, begin by determining the number of electrons from the periodic table. 11k views 8 years ago. Chlorine is a diatomic molecule and contains only two chlorine atoms. In the periodic table, it is the 3rd period and p block with the atomic number 17. The valence electrons are the electrons in the outermost shell. It helps chemists understand the bonding behavior of chlorine and how it interacts with other elements to form compounds. Take note of the electron configuration for reference and follow the three fundamental rules:. The chlorine molecule contains two chlorine atoms. Let us first start with determining the number of protons in chlorine. In this video we'll look at the atomic structure and bohr model for the chlorine atom (cl). Web to illustrate the chlorine orbital diagram, begin by determining the number of electrons from the periodic table. There are several steps to complete. Electrons are the negatively charged particles that orbit the nucleus of an atom. Lewis structures show all of the valence electrons in an atom or molecule. It is very easy to draw the cl 2 lewis structure. Web construct a qualitative molecular orbital diagram for chlorine, cl 2. Chlorine is a diatomic molecule and contains only two chlorine atoms. Swimming pool searvcice flat concept. So draw the nucleus of chlorine atom as follows: It helps chemists understand the bonding behavior of chlorine and how it interacts with other elements to form compounds. Each step of drawing is explained in detail in this tutorial. Determine total electrons pairs as lone pairs and bonds. The number of protons for any atom is equal to the atomic number of that atom. The nucleus of a chlorine atom contains 17 protons and 18 neutrons. How to draw the atomic model for chlorine. In the periodic table, it is the 3rd period and p block with the atomic number 17. Web steps of drawing lewis structure of ccl 4. Chlorine is the second lightest halogen and is represented as cl.
Chlorine 제이위키
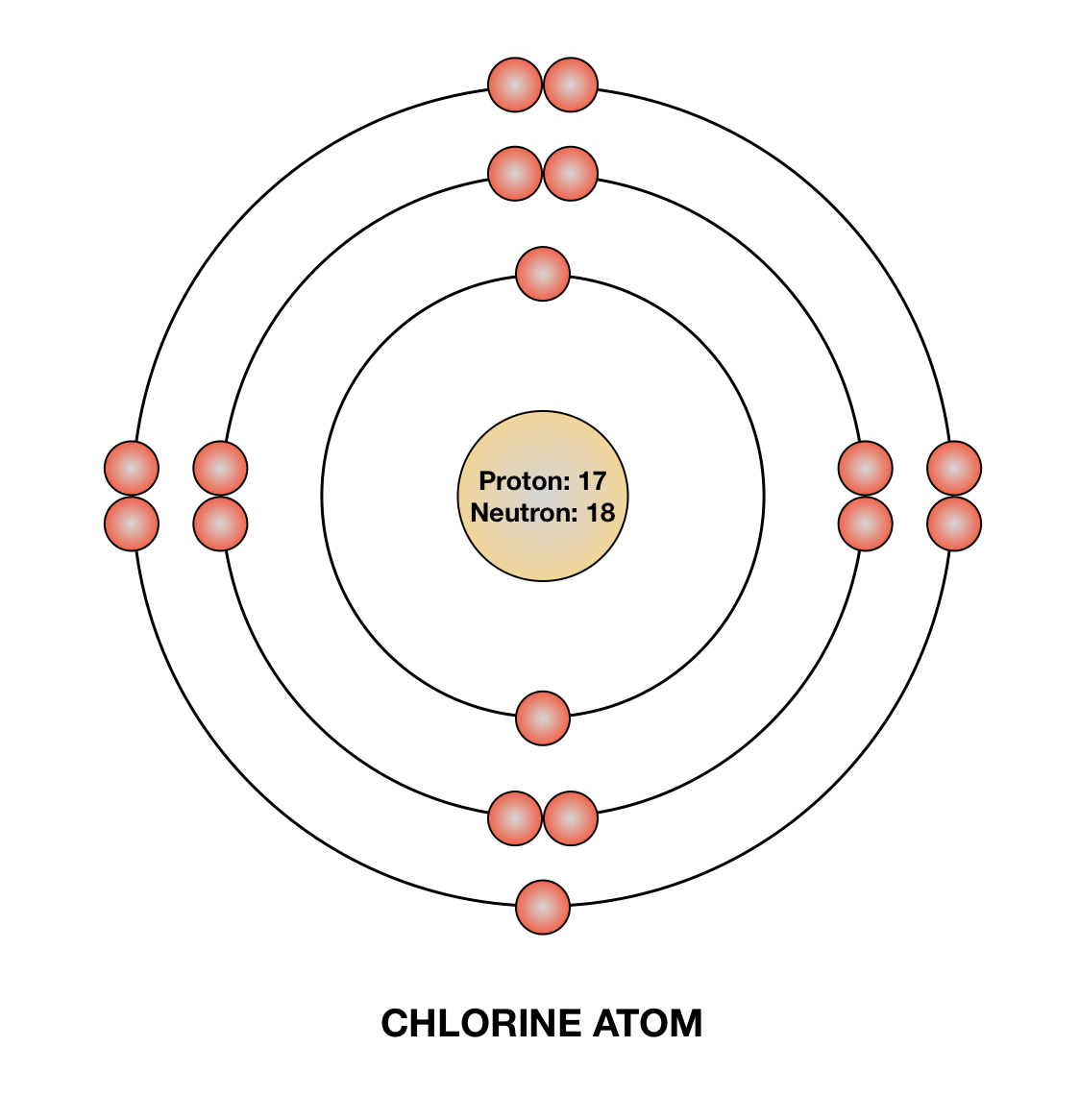
Draw a Bohr diagram of chlorine. Quizlet
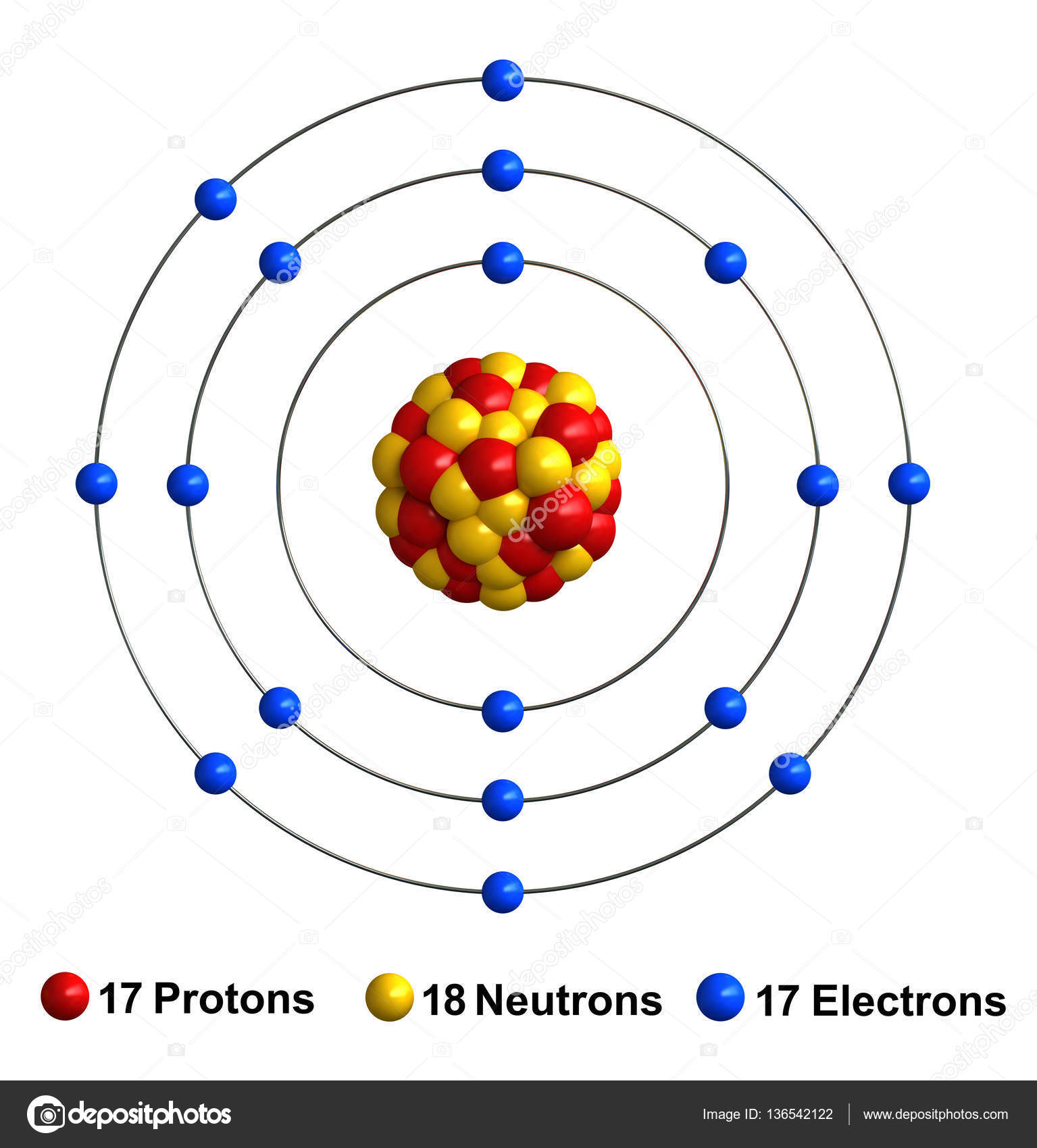
3d Bohr Model Of Chlorine
Determine The Total Number Of Valence Electrons In Chlorine Molecule.
There Are Several Steps To Complete The Lewis Structure Of Ccl 4.
The Nature Bond And Place Of Atoms In A Molecule Is Determined By The Lewis Dot Structure Of That Molecule, The Electronic Configuration Of Chlorine Is As Follows,
A Lewis Structure Is A Way To Show How Atoms Share Electrons When They Form A Molecule.
Related Post: