Drawing Molecular Orbital Diagrams
Drawing Molecular Orbital Diagrams - Draw two lines to create three columns. In the case of n2, the atomic orbitals involved are the 2p orbitals of the nitrogen atoms. For a diatomic molecule, the atomic orbitals of one atom are shown on the left, and those of the other atom are shown on the right. In the first column, draw the atomic diagram for your first element. We saw two simple mo diagrams in the section on h 2. These steps may then be extrapolated to construct more difficult polyatomic diagrams. Molecular orbital theory is a more sophisticated model for understanding the nature of chemical bonding. Web drawing molecular orbital diagrams is one of the trickier concepts in chemistry. Web about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features nfl sunday ticket press copyright. They also demonstrate the molecule’s bond order, or how many bonds are shared between the two atoms. Determine the atomic orbitals of your atoms. Once you’ve drawn a molecule, you can click the 2d to 3d button to convert the molecule into a 3d model which is then. What do the two structures tell you about electron pairing? In the third column, draw the atomic diagram for your second element. We saw two simple mo diagrams in. They also demonstrate the molecule’s bond order, or how many bonds are shared between the two atoms. In the case of n2, the atomic orbitals involved are the 2p orbitals of the nitrogen atoms. Web molecular orbital energy diagrams. Web molecular orbital diagrams. It helps to determine the bond order, identify the nature of chemical bonding, and provide insights into. Once you’ve drawn a molecule, you can click the 2d to 3d button to convert the molecule into a 3d model which is then. Web the molecular orbital theory allows one to predict the distribution of electrons in a molecule which in turn can help predict molecular properties such as shape, magnetism, and bond order. Web learn to draw molecular. 891k views 3 years ago new ap & general chemistry video playlist. Web the objective of this wiki is to provide readers with the fundamental steps in constructing simple homonuclear and heteronuclear diatomic molecular orbital diagrams. Web to understand the bonding of a diatomic molecule, a molecular orbital diagram is used. Web a molecular orbital diagram, or mo diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (lcao) method in particular. Web the molecular orbital theory allows one to predict the distribution of electrons in a molecule which in turn can help predict molecular properties such as shape, magnetism, and bond order. The relative energy levels of atomic and molecular orbitals are typically shown in a molecular orbital diagram (figure 8.34). In the first column, draw the atomic diagram for your first element. Web molecular orbital energy diagrams. General chemistry supplement (eames) molecular orbital theory. Mo diagrams can be used to determine a molecule’s magnetic properties and how they change with ionisation. By providing a visual representation of electron distribution and energy levels, these diagrams offer insights into stability, reactivity, properties, and electronic transitions. Web in summary, drawing a molecular orbital diagram for n2 is essential for understanding the bonding, electronic structure, and properties of the nitrogen molecule. It helps to determine the bond order, identify the nature of chemical bonding, and provide insights into the electronic configuration of n2. Web about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features nfl sunday ticket press copyright. Compounds that have all of their electrons paired are referred to as diamagnetic. Construct mo diagrams for simple diatomic molecules and/or compounds.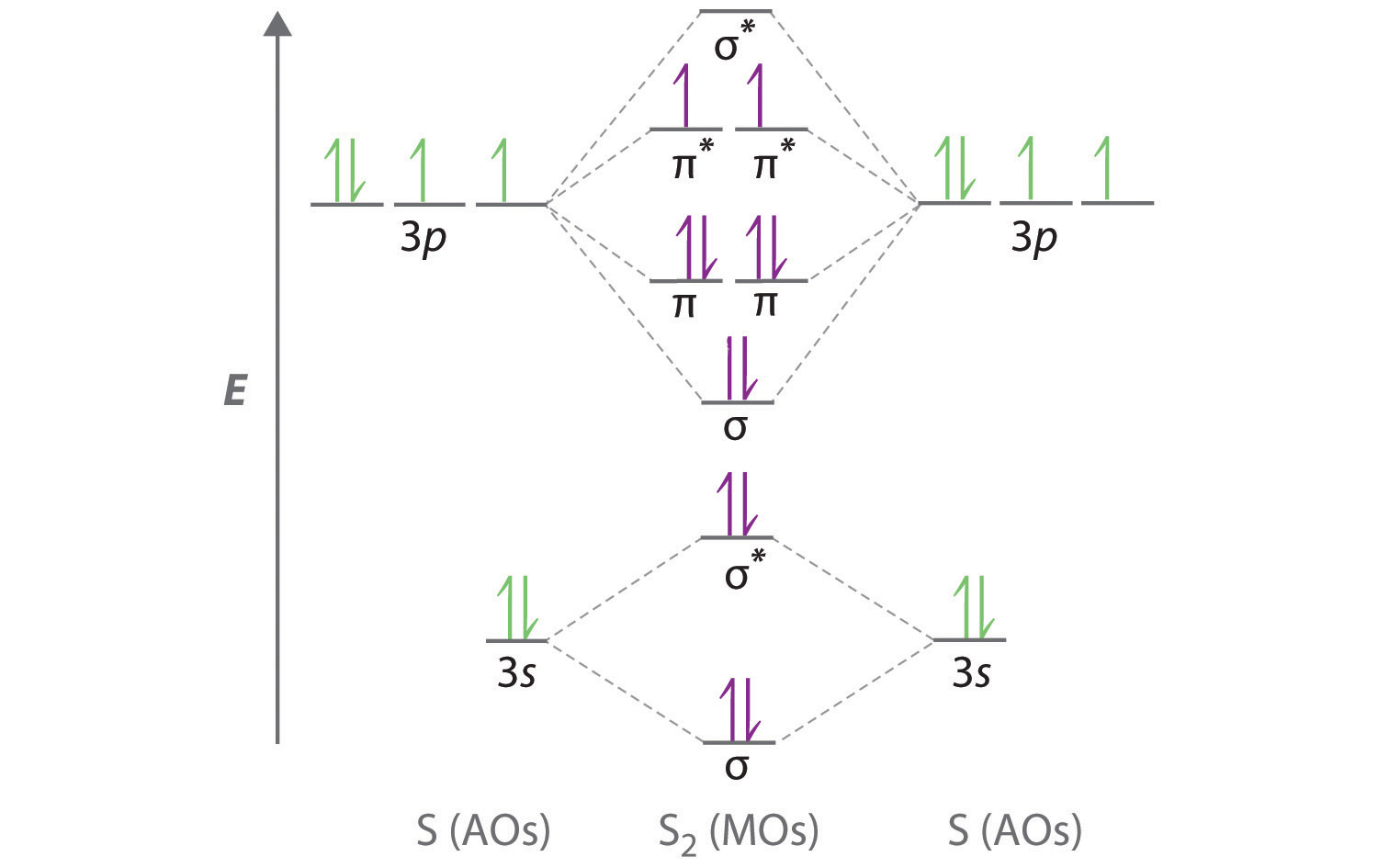
Molecular Orbital Diagram For Cl2

6.6 3D Representation of Orbitals Chemistry LibreTexts

Molecular Orbital Diagrams simplified by Megan Lim Medium
Web Molecular Orbital Diagrams Are Complex, Involving Two Additional Orbitals, Electronegativity, Atomic Symmetries And Atomic Energies.
It Describes The Formation Of.
For A Diatomic Molecule, The Atomic Orbitals Of One Atom Are Shown On The Left, And Those Of The Other Atom Are Shown On The Right.
What Do The Two Structures Tell You About Electron Pairing?
Related Post: