Drawing Lewis Structures Practice
Drawing Lewis Structures Practice - A review of general chemistry. A lewis structure is a diagram that shows the chemical bonds between atoms in a molecule and. Web the organic chemistry tutor. Web lewis structures test + practice problems flashcards | quizlet. 2.1m views 6 years ago new ap & general chemistry video playlist. One of the fundamental differences of organic chemistry from general. You need to register to access the answers and solutions for all the. Web practice drawing lewis structures with answers and explanation. (please note, none of the solutions are using the expanded octet rule or formal charges) h 2. 1) calculate the total number of valence electrons for the molecule. 2.1m views 6 years ago new ap & general chemistry video playlist. Write lewis structures for the following: Web things to remember 1. Web practise drawing the lewis structure of molecules using the exercises below. Choose the number, type, and. Web lewis structures and the octet rule. This chemistry video provides a basic. Web answer the following questions and check your answers below. Web a video tutorial for how to draw lewis structures in five steps. Be sure you know how to draw correct lewis dot. The video covers the basic lewis structures you'll see in an introductor. Web things to remember 1. Ethanethiol, c a 2 h a 6 s , is a clear liquid with a strong odor. The first is surrounded by three h atoms above, below, and to the side. You need to register to access the answers and solutions for all. The example is for the nitrate ion. Web a video tutorial for how to draw lewis structures in five steps. Web the organic chemistry tutor. Web lewis structures and the octet rule. (please note, none of the solutions are using the expanded octet rule or formal charges) h 2. Web drawing lewis structures for molecules with one central atom: Web lewis structures test + practice problems flashcards | quizlet. One of the fundamental differences of organic chemistry from general. In other cases choose the element that is the least. The video covers the basic lewis structures you'll see in an introductor. For the following molecules or ions (where the central atom. Choose the number, type, and. Web things to remember 1. A review of general chemistry. Be sure you know how to draw correct lewis dot. Web draw the lewis dot structure for each of the following polyatomic ions: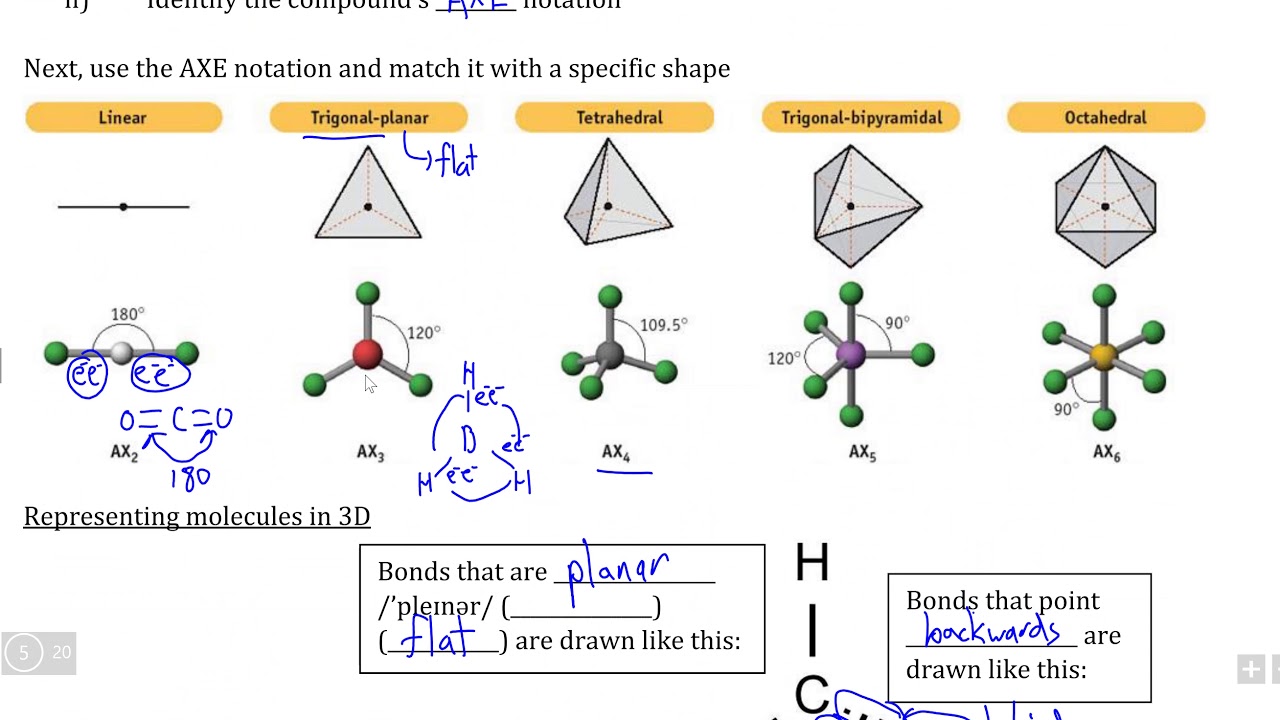
Structural Chemistry II, Video II Drawing 3D Lewis Structures I

3 Ways to Draw Lewis Dot Structures wikiHow

Lewis Electron Dot Structure Calculator
The Video Covers The Basic Lewis Structures You'll See In An Introductory Chemistry Class.
Select Answers To See The Correct Drawings.
Click The Card To Flip 👆.
A Lewis Structure Is A Diagram That Shows The Chemical Bonds Between Atoms In A Molecule And.
Related Post: