Drawing Hybridized Orbitals
Drawing Hybridized Orbitals - What is the difference between a resonance structure and a resonance hybrid structure? Web steps to draw hybrid orbital diagrams for a molecule. Read through the provided information, and sketch the lewis dot diagram of the provided compound. In sp³ hybridization, one s orbital and three p orbitals hybridize to form four sp³ orbitals, each consisting of 25% s character and 75% p character. This type of hybridization is required whenever an atom is surrounded by four groups of electrons. This 109.5 o arrangement gives tetrahedral geometry (figure 4). What is the hybridization around the central carbon atom in co 2 ? Describe the hybrid orbitals used in the formation of bonding for each atom in some carbon containing compounds. Hybrid orbitals overlap to form σ bonds. In the following sections, we shall discuss the common types of hybrid orbitals. Apparently forming the four bonds, in the configuration we call the sp3 hybrid orbitals, is a low energy state of the carbon in that situation. The three sp 2 hybrids are arranged with trigonal planar geometry, pointing to the three corners of an equilateral triangle, with angles of 120°between them. Hybrid orbitals overlap to form σ bonds. In sp³ hybridization,. Any molecule can be formed using linear combination of atomic orbitals (lcao). Web the two sp hybrid orbitals are oriented 180° away from each other, perpendicular to the two remaining p orbitals (red/blue). How do electrons in atomic valence orbitals form bonds? This 109.5 o arrangement gives tetrahedral geometry (figure 4). Any atom with the principal orbital quantum number, n=30. Web steps to draw hybrid orbital diagrams for a molecule. Unhybridized orbitals overlap to form π bonds. Archived lecture notes #2 (pdf), section 3. Apparently forming the four bonds, in the configuration we call the sp3 hybrid orbitals, is a low energy state of the carbon in that situation. Lewis dot diagrams and vsepr are powerful tools to think about. Web steps to draw hybrid orbital diagrams for a molecule. In the following sections, we shall discuss the common types of hybrid orbitals. Web draw any atom: Any atom with the principal orbital quantum number, n=30. What is the difference between a resonance structure and a resonance hybrid structure? Want to join the conversation? Unhybridized orbitals overlap to form π bonds. Lewis dot diagrams and vsepr are powerful tools to think about electronic configuration and molecular shape, but neither tool offers a way to predict bond stability. Explain the concept of atomic orbital hybridization. Learn for free about math, art, computer programming, economics, physics, chemistry, biology, medicine, finance, history, and more. How do electrons in atomic valence orbitals form bonds? Web the formation of hybrid atomic orbitals can be viewed as occurring via promotion of an electron from a filled ns 2 subshell to an empty np valence orbital, followed by hybridization, the combination of the orbitals to give a new set of (usually) equivalent orbitals that are oriented properly to form bonds. Apparently forming the four bonds, in the configuration we call the sp3 hybrid orbitals, is a low energy state of the carbon in that situation. The hybrid orbital model helps to explain this. Hybrid orbitals overlap to form σ bonds. Describe the hybrid orbitals used in the formation of bonding for each atom in some carbon containing compounds.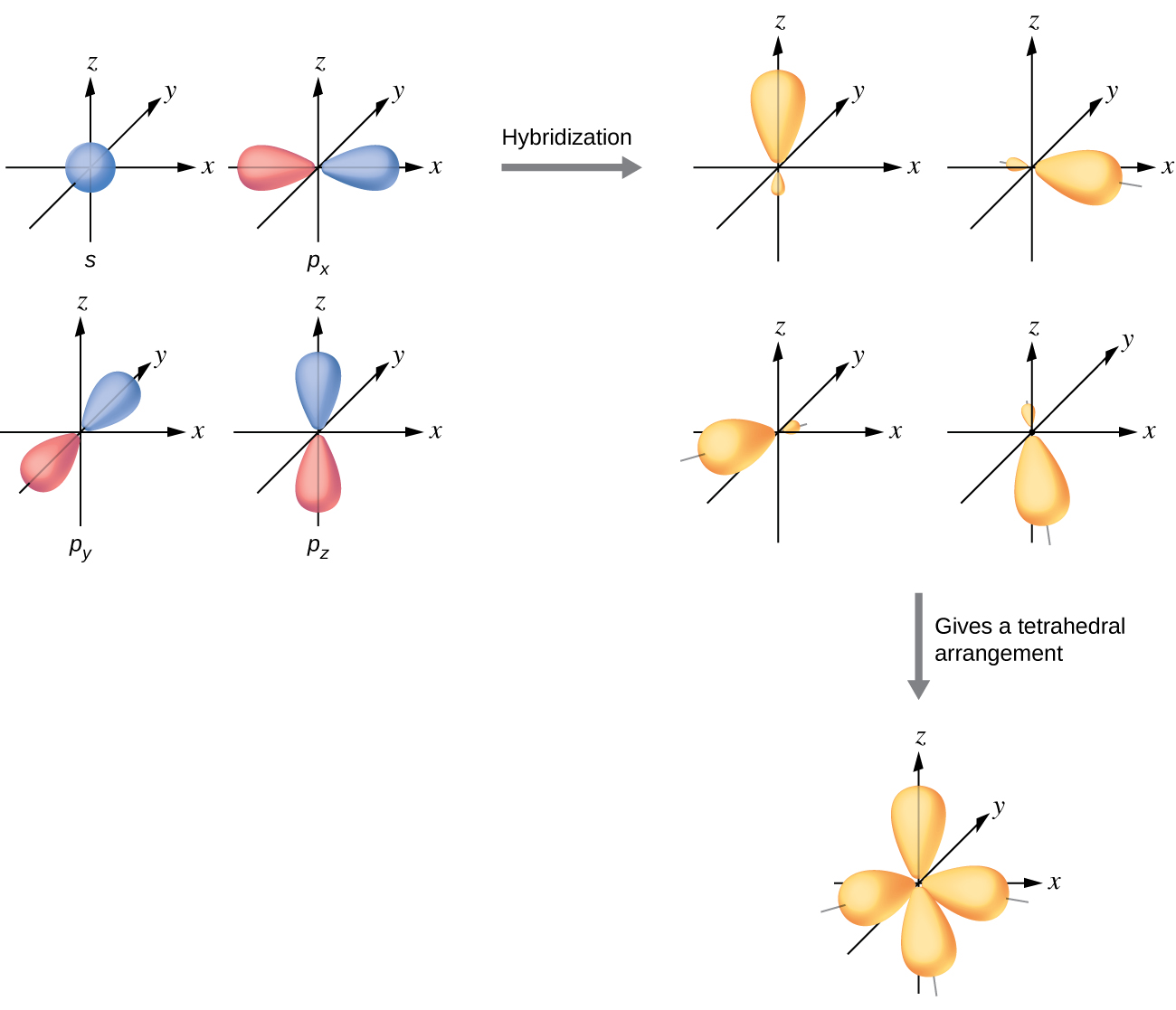
8.2 Hybrid Atomic Orbitals Chemistry
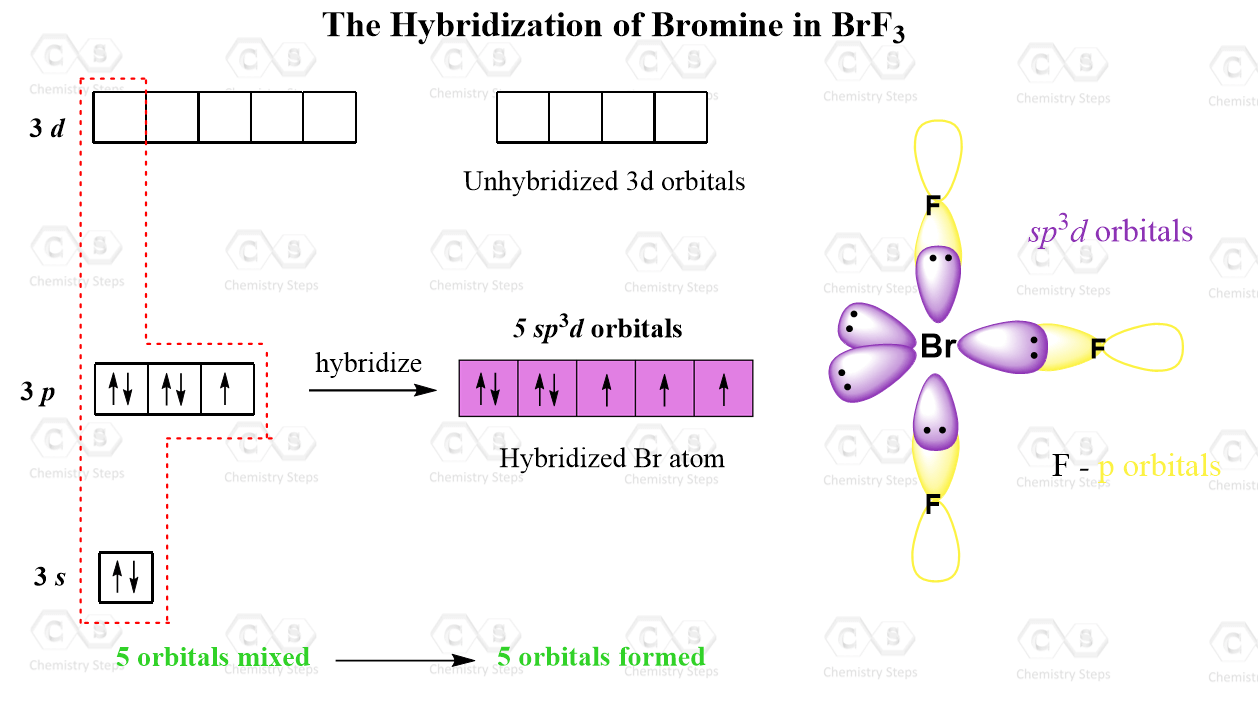
Hybridization of Atomic Orbitals Chemistry Steps
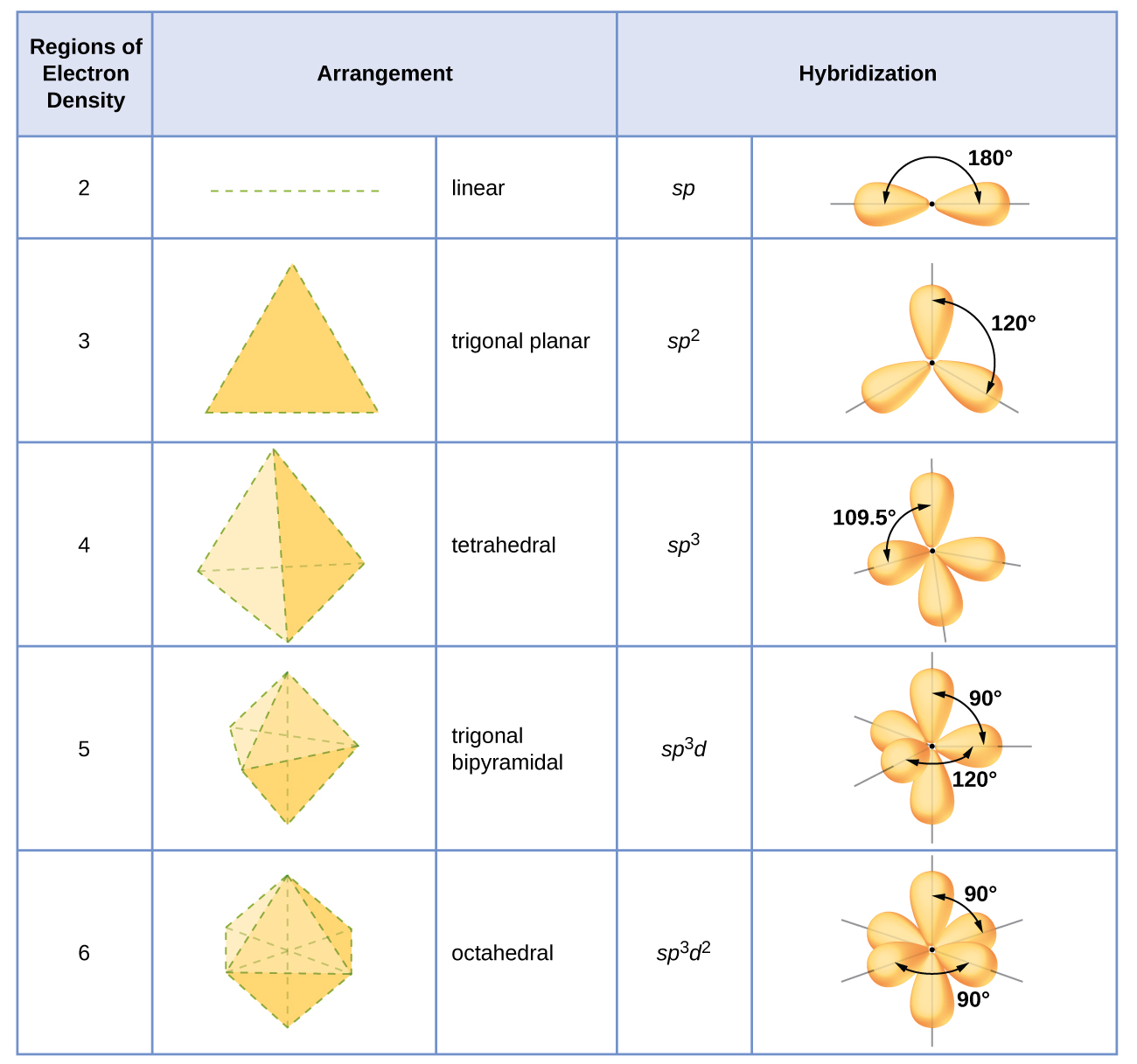
8.2 Hybrid Atomic Orbitals Chemistry LibreTexts
Recall The Valence Electron Configuration Of A Carbon Atom:
Unhybridized Orbitals Overlap To Form Π Bonds.
This 109.5 O Arrangement Gives Tetrahedral Geometry (Figure 4).
Web By Hybridizing Its 2 S And 2 P Orbitals, It Can Form Four Sp3 Hybridized Orbitals That Are Equal In Energy.
Related Post: