Drawing Hybridization Orbitals
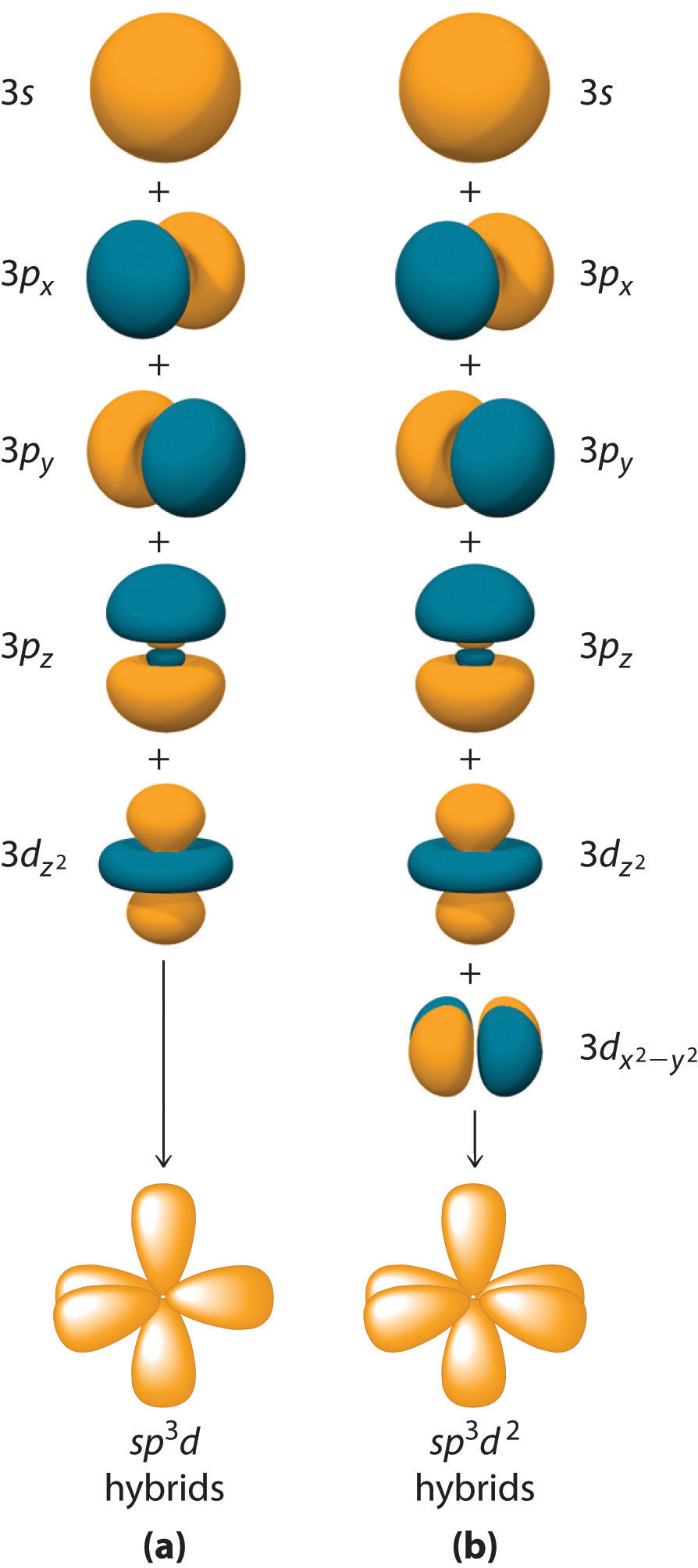
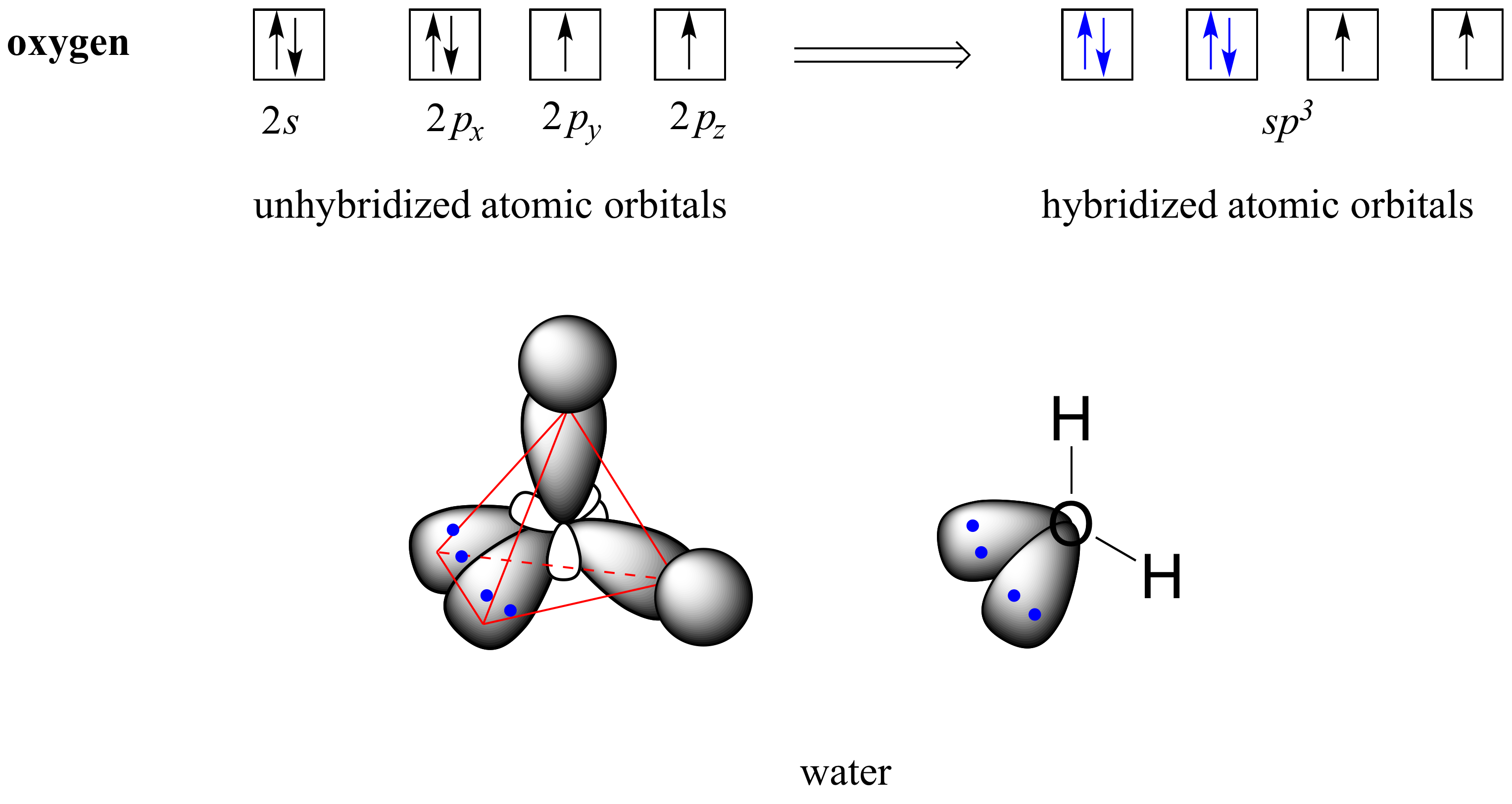
Drawing Hybridization Orbitals - Web hybridization examples and hybrid orbitals: Explain the concept of atomic orbital hybridization. Web draw orbital box diagrams showing how combinations of an atomic s orbital and various numbers of p orbitals create sp, sp 2, and sp 3 hybrid orbitals. This type of hybridization is required whenever an atom is surrounded by four groups of. Web figure 1.16 sp hybridization. Web energy changes occurring in hybridization. Web a set of hybrid orbitals is generated by combining atomic orbitals. Thinking in terms of overlapping. In sp³ hybridization, one s orbital and three p orbitals hybridize to form four sp³ orbitals, each consisting of 25% s character and 75% p character. Determine the hybrid orbitals associated with various molecular geometries. Hybridization of an s orbital with two p orbitals (p x and p y) results in three sp 2 hybrid orbitals that are oriented at 120. 1.9m views 3 years ago new ap & general chemistry video playlist. Web explain the concept of atomic orbital hybridization. Determine the hybrid orbitals associated with various molecular geometries. Explain the concept of atomic. Explain the concept of atomic orbital hybridization. Determine the hybrid orbitals associated with various molecular geometries. This type of hybridization is required whenever an atom is surrounded by four groups of. Web hybridization examples and hybrid orbitals: Web in chemistry, orbital hybridisation (or hybridization) is the concept of mixing atomic orbitals to form new hybrid orbitals (with different energies, shapes,. Web explain the concept of atomic orbital hybridization. Web this organic chemistry video tutorial provides a basic introduction into valence bond theory and hybrid atomic orbitals. The two sp hybrid orbitals are oriented 180° away from each other, perpendicular to the two remaining p orbitals (red/blue). Web in chemistry, orbital hybridisation (or hybridization) is the concept of mixing atomic orbitals. This organic chemistry video tutorial explains the hybridization of atomic orbitals. Web a set of hybrid orbitals is generated by combining atomic orbitals. Web this organic chemistry video tutorial provides a basic introduction into valence bond theory and hybrid atomic orbitals. Web explain the concept of atomic orbital hybridization. This type of hybridization is required whenever an atom is surrounded by four groups of. Web draw orbital box diagrams showing how combinations of an atomic s orbital and various numbers of p orbitals create sp, sp 2, and sp 3 hybrid orbitals. In sp³ hybridization, one s orbital and three p orbitals hybridize to form four sp³ orbitals, each consisting of 25% s character and 75% p character. The two sp hybrid orbitals are oriented 180° away from each other, perpendicular to the two remaining p orbitals (red/blue). Web in chemistry, orbital hybridisation (or hybridization) is the concept of mixing atomic orbitals to form new hybrid orbitals (with different energies, shapes, etc., than the component. Determine the hybrid orbitals associated with various molecular geometries. Hybridization of an s orbital with two p orbitals (p x and p y) results in three sp 2 hybrid orbitals that are oriented at 120. Web energy changes occurring in hybridization. Web figure 1.16 sp hybridization. Explain the concept of atomic orbital hybridization. Web hybridization and atomic orbitals. Web hybridization examples and hybrid orbitals: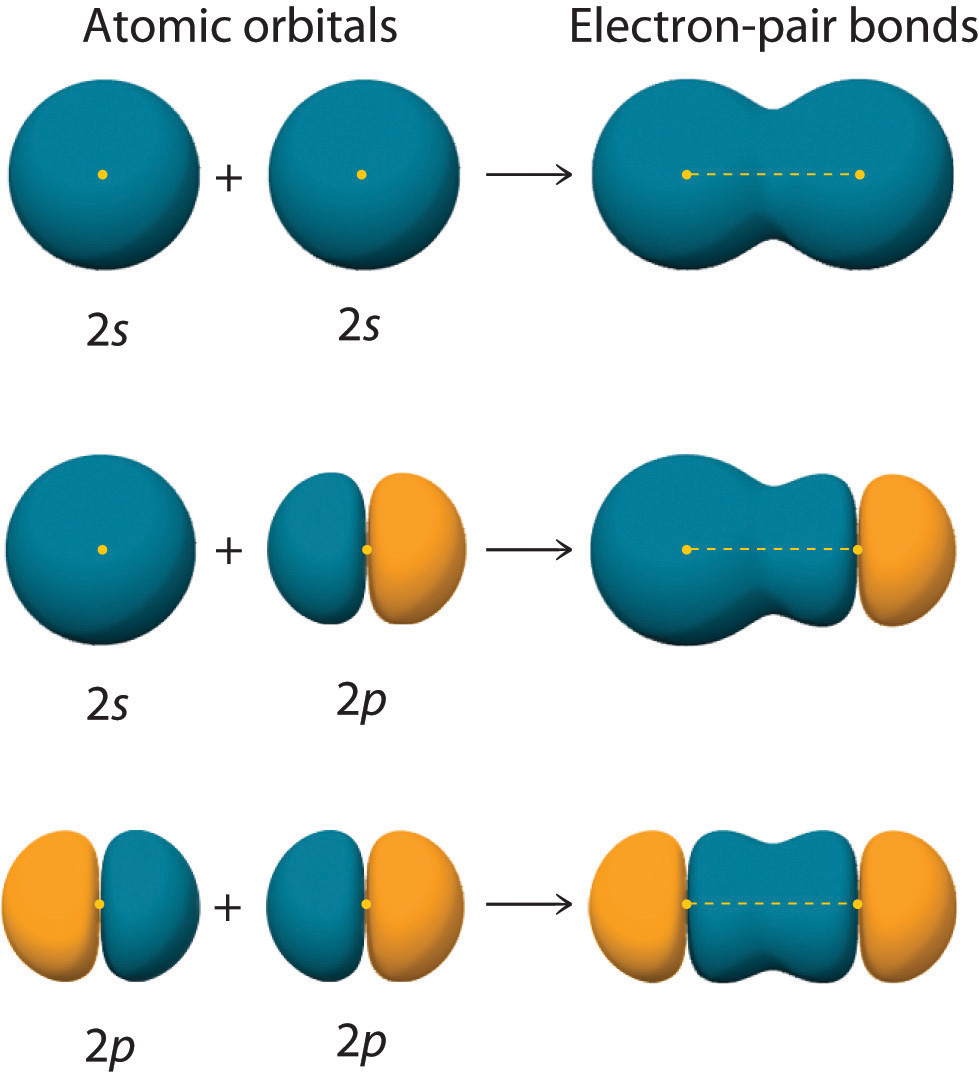
Chapter 6.2 Hybrid Orbitals Chemistry LibreTexts
9.5 Hybrid Orbitals Chemistry LibreTexts
2.2 Hybrid orbitals Chemistry LibreTexts
Web The Formation Of Hybrid Atomic Orbitals Can Be Viewed As Occurring Via Promotion Of An Electron From A Filled Ns 2 Subshell To An Empty Np Valence Orbital,.
1.9M Views 3 Years Ago New Ap & General Chemistry Video Playlist.
Thinking In Terms Of Overlapping.
Hybridization Was Introduced To Explain Molecular Structure When The Valence Bond Theory Failed To Correctly Predict Them.
Related Post: