Drawing Dipole Moments
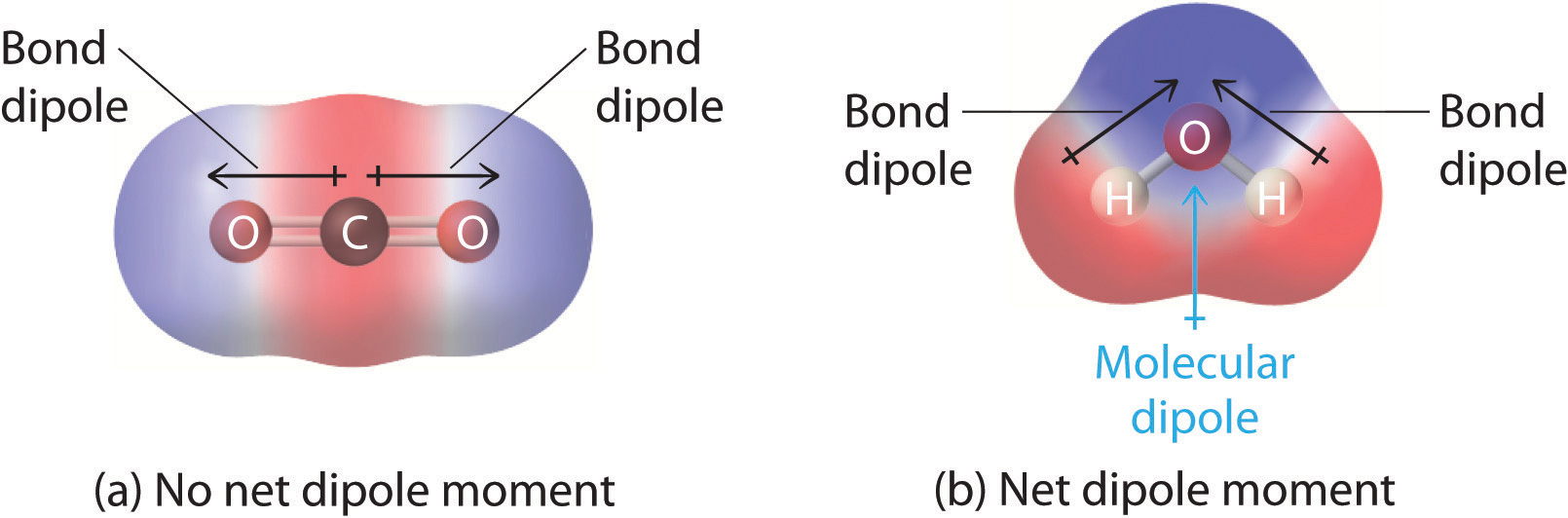
Drawing Dipole Moments - In general, the magnitude of the dipole moment is given by the product of the magnitude of the charge and the distance between the charges. For diatomic molecules, there is only one bond, so its bond dipole moment determines the molecular polarity. Predict whether a molecule will possess a dipole moment from the molecular formula or structure. Web it is relatively easy to measure dipole moments: If you get stuck, try asking another group for help. Web predicting the direction of a dipole moment. From in between the hydrogen atoms to the oxygen atom. Looking at the electronegativity and shape of the h2o molecule tells you how the arrow depicts the polarity: Polar molecules increase the charge stored on the plates, and the dipole moment can be obtained (i.e., via the capacitance of the system). Use the presence or absence of a dipole moment as an aid to deducing the structure of a given compound. You should try to answer the questions without referring to your textbook. Work in groups on these problems. When you place a molecule with an electric dipole in an electric field, a force acts to turn the molecule so that the positive and negative ends line up with the field. Web a dipole moment occurs in covalent bonds between atoms. It is denoted by the greek letter ‘µ’. If you get stuck, try asking another group for help. After completing this section, you should be able to. Mathematically, dipole moment (µ) = charge (q) * distance of separation (r) Explain how dipole moments depend on both molecular shape and bond polarity. Web the overall dipole moment of a molecule depends on the individual bond dipole moments and how they are arranged. Web drawing dipole moments. In general, the magnitude of the dipole moment is given by the product of the magnitude of the charge and the distance between the charges. Mathematically, dipole moment (µ) = charge (q) * distance of separation. We start by looking at a water molecule: Consider a diatomic molecule ab. For each of the following, determine if the molecule would have a dipole moment (polar or nonpolar): Web a dipole moment occurs in covalent bonds between atoms of differing electronegativities. The direction of the dipole moment is shown by the following sign in which the arrow points to the partially negatively charged end of the dipole: The vector sum of these arrows will be the overall dipole moment for the molecule. As long as you label the delta negative and the delta positive, i'm sure you'll be fine, but i think we are supposed to draw them positive to negative. Web explain how dipole moments depend on both molecular shape and bond polarity. Dipole moments arise from differences in electronegativity. From in between the hydrogen atoms to the oxygen atom. Web drawing dipole moments. Web dipole moments occur when there is a separation of charge. Work in groups on these problems. Learn about carbon dioxide's dipole moment, see how bond dipoles work and understand what molecular polarity is to help determine whether a molecule is polar. (b) in contrast, water is polar because the oh bond moments do not cancel out. Just place a substance between charged plates (figure \(\pageindex{2}\));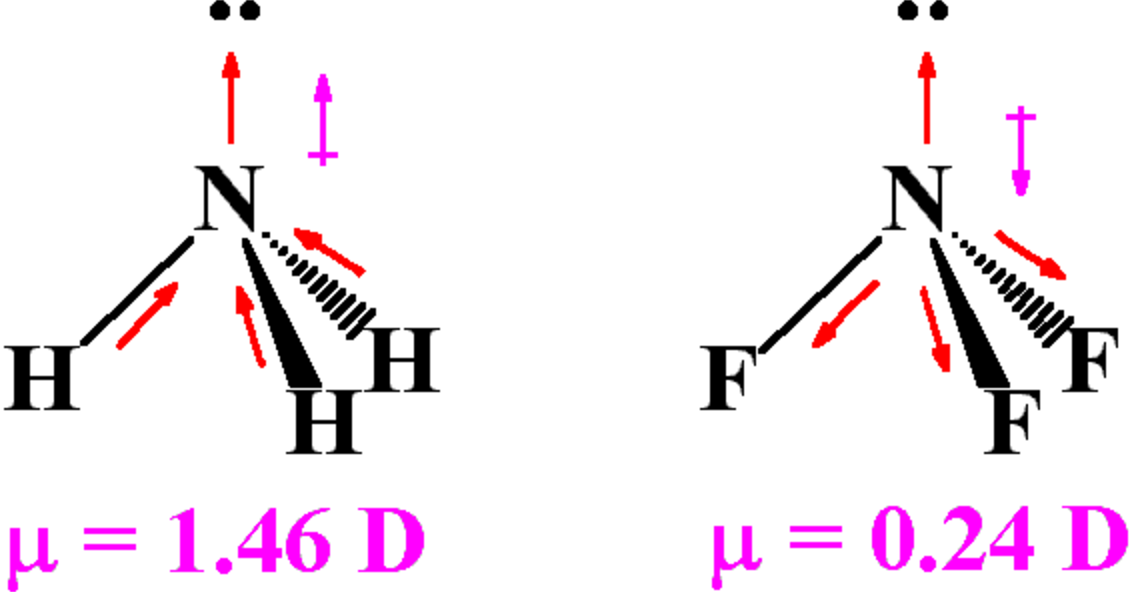
Dipole Moments Chemical Bonding and Molecular Structure, Chemistry
2.2 Polar Covalent Bonds Dipole Moments Chemistry LibreTexts
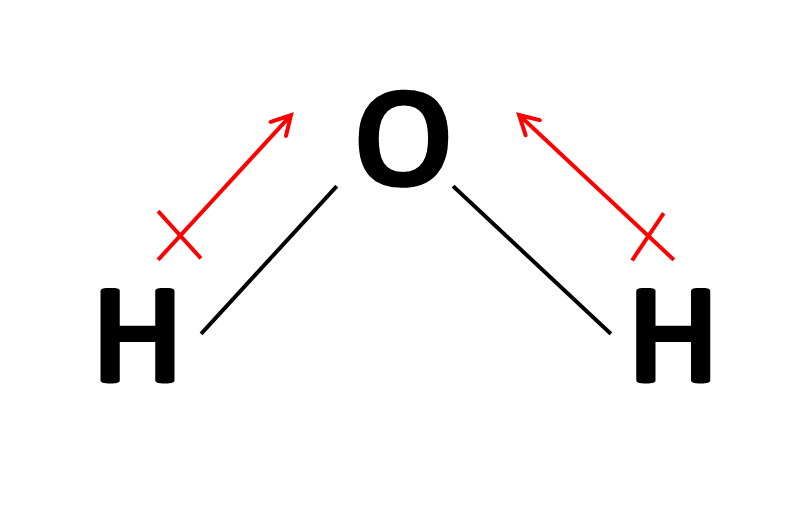
How do we draw the dipole moment of a water molecule? Socratic
Predict Whether A Molecule Will Possess A Dipole Moment, Given Only Its Molecular Formula Or Kekulé Structure.
Mathematically, Dipole Moment (Μ) = Charge (Q) * Distance Of Separation (R)
Web The Overall Dipole Moment Of A Molecule Depends On The Individual Bond Dipole Moments And How They Are Arranged.
Μ = Q × D.
Related Post: