Drawing A Heating Curve
Drawing A Heating Curve - Web to plot a heating curve, the temperature of the substance and the amount of heat added to the substance should be recorded at regular intervals. Web the heating curve for water shows how the temperature of a given quantity of water changes as heat is added at a constant rate. Web it covers how to draw a heating curve for a specific substance when given information about that substance (i.e. In addition to the two paragraphs describing the state changes, a heating curve graph is included. Web how to draw a heating curve? To calculate the energy changes that accompany phase changes. Web heat ice from −15 °c to 0 °c. The sample is initially ice at 1 atm and −23°c; Describe the processes represented by typical heating and cooling curves, and compute heat flows and enthalpy changes accompanying these processes. Graph functions, plot points, visualize algebraic equations, add sliders, animate graphs, and more. Web in this video, we will be studying how to read and draw a heating curve. Changes in state and heating curves. In this section, we continue analyzing phase diagrams (plots of pressure vs. Web heat ice from −15 °c to 0 °c. Web how to draw a heating curve? Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. A heating curve can be constructed by plotting a. Explain the construction and use of a typical phase diagram. Web dennyd's interactive graph and data of heating curve graph is a scatter chart, showing col2; Web how to draw a. On the workbook, no.5 on the quiz preparation, it asks us to draw a heating curve based on the enthalpy of fusion, enthalpy of vaporisation, and the heat capacity for solid, liquid and gas phases. Web dennyd's interactive graph and data of heating curve graph is a scatter chart, showing col2; In addition to the two paragraphs describing the state. Graph functions, plot points, visualize algebraic equations, add sliders, animate graphs, and more. Gce chemistry topic 2.1 kinetic particle theory this video provide a step by. On the workbook, no.5 on the quiz preparation, it asks us to draw a heating curve based on the enthalpy of fusion, enthalpy of vaporisation, and the heat capacity for solid, liquid and gas phases. The experiment described above can be summarized in a. Web a heating curve is constructed by measuring the temperature of a substance as heat is added at constant pressure. A heating curve can be constructed by plotting a. Describe the processes represented by typical heating and cooling curves, and compute heat flows and enthalpy changes accompanying these processes. 191k views 12 years ago every video. Web once all the liquid has completely boiled away, continued heating of the steam (since the container is closed) will increase its temperature above \(100^\text{o} \text{c}\). During a phase change, the temperature of the water remains constant, resulting in a plateau on the graph. Q = m × c × δ t (see previous chapter on thermochemistry). This is because the added heat is used to break the intermolecular forces holding the particles together and convert the substance from one phase to another. 4.8k views 3 years ago matter & the atom. The sample is initially ice at 1 atm and −23°c; Explain the construction and use of a typical phase diagram. Energy) that you learned about earlier in the semester.
Heating and Cooling Curves — Overview & Examples Expii
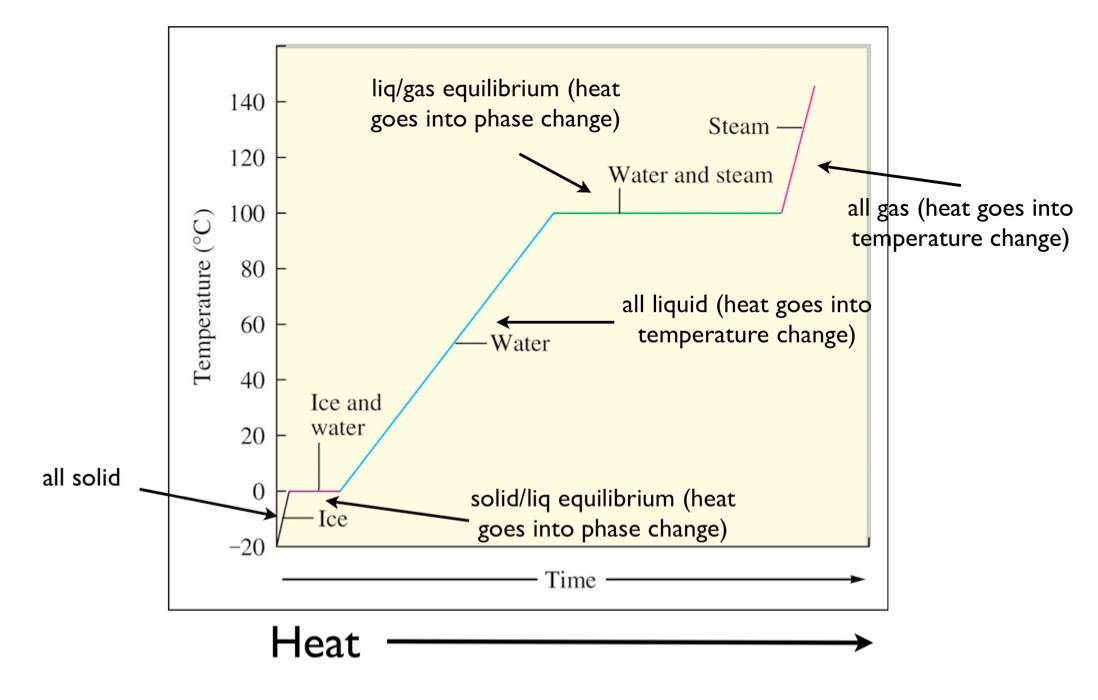
heating curve
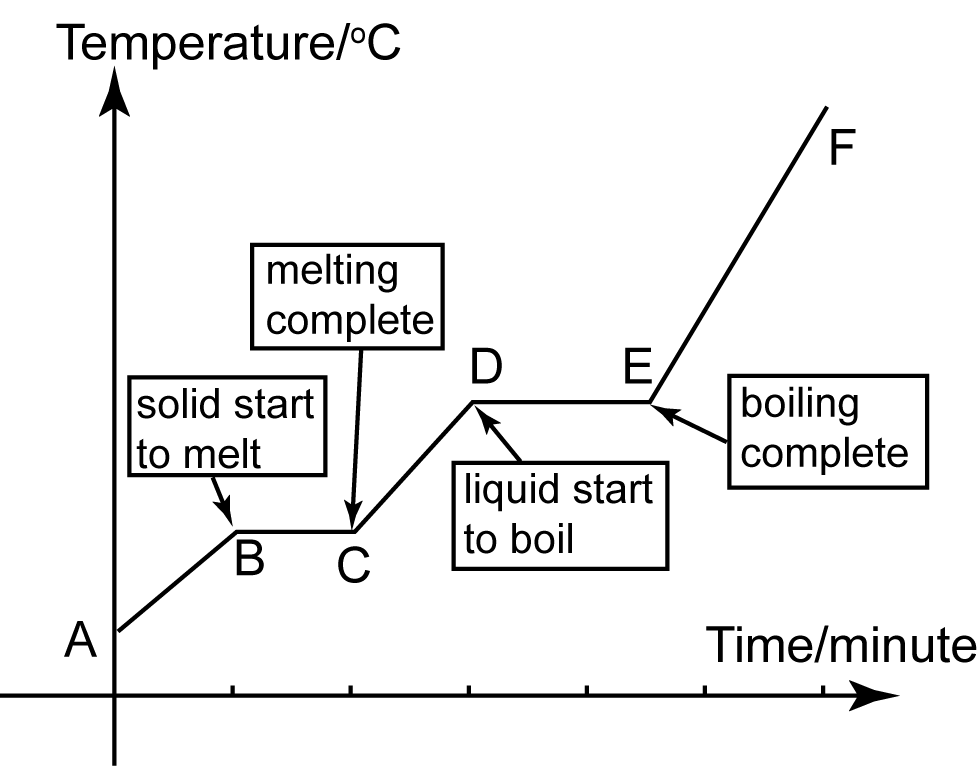
Heating Curve SPM Chemistry Form 4/Form 5 Revision Notes
To Calculate The Energy Changes That Accompany Phase Changes.
Graph Functions, Plot Points, Visualize Algebraic Equations, Add Sliders, Animate Graphs, And More.
Web Explore Math With Our Beautiful, Free Online Graphing Calculator.
During A Plateau, The Temperature Remains Constant Despite The Continuous Addition Of Heat.
Related Post: