Draw Valine At Physiological Ph
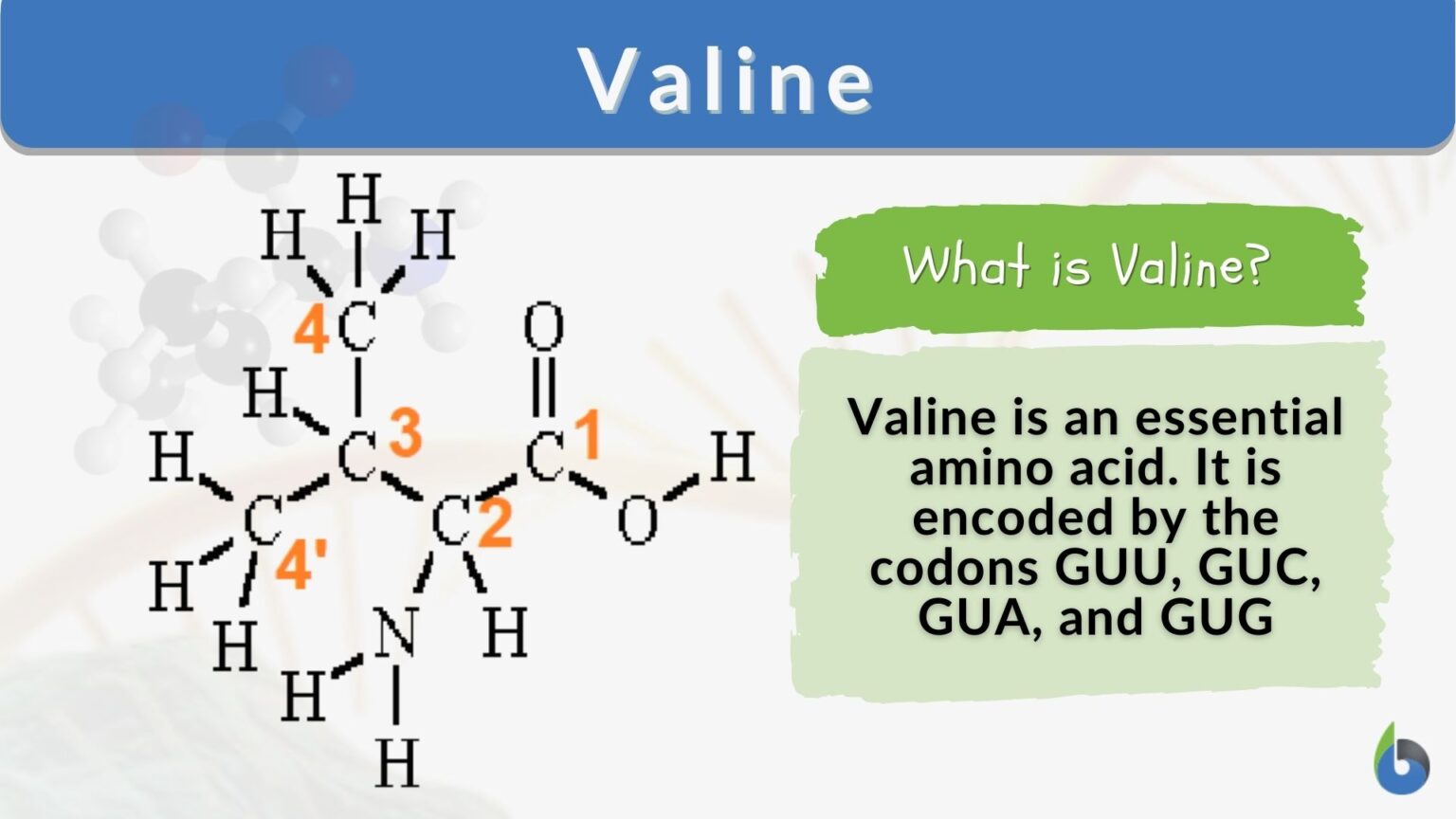
Draw Valine At Physiological Ph - We were talking about it at ph of one, which is really, really. Some groups are hydrophobic (e.g., branched chain and aromatic amino acids) and some hydrophilic (most others). Example 26.1 draw the structure for the anion formed when glycine (at neutral ph) reacts with a base. Web introduction to general, organic and biochemistry. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. The particular ph at which a given amino acid exists in solution as a zwitterion is called the isoelectric point (pi). Therefore the \(\ce{h^+}\) will add to the carboxylate ion and neutralize the negative charge. A ph at this level is ideal for many biological processes, one of the most important being the oxygenation of blood. Low ph pi high ph d. Draw the predominant form of valine when the ph = 1.0 c. Web in both circumstances, the amino acid acts to maintain the ph of the system—that is, to remove the added acid (h +) or base (oh −) from solution. We were talking about it at ph of one, which is really, really. Draw the predominant form of a given amino acid in a solution of known ph, given the isoelectric. Web at ph = 3.52, the \(\ce{h^+}\) concentration is high (low ph = more acidic = more \(\ce{h^+}\)). The particular ph at which a given amino acid exists in solution as a zwitterion is called the isoelectric point (pi). Web draw the structure for the cation formed when valine (at neutral ph) reacts with an acid. It must be obtained. Therefore the \(\ce{h^+}\) will add to the carboxylate ion and neutralize the negative charge. Draw the structure for the anion formed when valine (at neutral ph) reacts with a base. Web introduction to general, organic and biochemistry. Web a tool that draws peptide primary structure and calculates theoretical peptide properties. Valine is essential in humans, meaning the body cannot synthesize. This problem has been solved! Example 26.1 draw the structure for the anion formed when glycine (at neutral ph) reacts with a base. Draw the structure for valine under the following conditions: Draw the structure for the anion formed when valine (at neutral ph) reacts with a base. We're talking about veiling this amino acid with on our group with two branch methyl groups like this. Web a tool that draws peptide primary structure and calculates theoretical peptide properties. In animal husbandry, supplementation with valine not only increases growth and reproductive performances, but also regulates gut microbiota and immune. Draw valine at physiological ph. After completing this section, you should be able to. Web in the absence of pathological states, the ph of the human body ranges between 7.35 to 7.45, with the average at 7.40. Some groups are hydrophobic (e.g., branched chain and aromatic amino acids) and some hydrophilic (most others). The amino acid structures as shown in lecture are the predominant forms at physiological ph (7.4). Web introduction to general, organic and biochemistry. A ph at this level is ideal for many biological processes, one of the most important being the oxygenation of blood. Why not a neutral number of 7.0 instead of a slightly alkaline 7.40? Draw the structure for the anion formed when valine (at neutral ph) reacts with a base.
Valine molecular structure. Valine skeletal chemical formula. Chemical

Valine Chemical Structure. Vector Illustration Hand Drawn Stock Vector
Valine Definition and Examples Biology Online Dictionary
You'll Get A Detailed Solution From A Subject Matter Expert That Helps You Learn Core Concepts.
Therefore The \(\Ce{H^+}\) Will Add To The Carboxylate Ion And Neutralize The Negative Charge.
We Were Talking About It At Ph Of One, Which Is Really, Really.
Draw The Predominant Form Of Valine When The Ph = 7.4 B.
Related Post: