Draw Two Resonance Structures Of The Cation Shown
Draw Two Resonance Structures Of The Cation Shown - Draw the partially expanded structures of the amino acids serine and alanine. Web (a) draw two resonance structures of the cation shown, shifting only one electron pair in each step. Introducing curved arrows, a tool for showing the movement of electrons between resonance structures. Draw two resonance structures of the cation shown, shifting only one electron pair in each. Web draw the resonance structures of molecules or ions that exhibit delocalization. Web draw all possible resonance structures for the following free radical: Sometimes one dot structures is not enough to completely describe a molecule or an ion, sometimes you need two or more, and here's an example: The electrons move over to here, to here and then finally, to here. Determine the relative stability of resonance structures using a set of rules. (a) draw two resonance structures of the cation shown, shifting only one electron pair in each step. In resonance structures, it does not require to show transformation of electrons by. (a) draw two resonance structures of the cation shown, shifting only one electron pair in each step. Web to draw all resonance structures, take the lewis structure we drawn by using vespr rule. The electrons move over to here, to here and then finally, to here. Determine. Introducing curved arrows, a tool for showing the movement of electrons between resonance structures. Draw two resonance structures of the cation shown, shifting only one electron pair in each. Be sure to include the formal charge on structures b and c. Use resonance structures to show electron delocalization. Be sure to include the formal charge on structures b and c. Sometimes one dot structures is not enough to completely describe a molecule or an ion, sometimes you need two or more, and here's an example: In resonance structures, it does not require to show transformation of electrons by. Web these two drawings are an example of what is referred to in organic chemistry as resonance contributors: Determine the relative stability. Two or more different lewis structures depicting the same. Some molecules have two or more chemically equivalent lewis electron structures, called resonance structures. Introducing curved arrows, a tool for showing the movement of electrons between resonance structures. Web to draw all resonance structures, take the lewis structure we drawn by using vespr rule. Use resonance structures to show electron delocalization. Web we draw our resonance brackets and go ahead and draw our other resonance structure for benzene. Determine the relative stability of resonance structures using a set of rules. Web draw the resonance structures of molecules or ions that exhibit delocalization. Web (a) draw two resonance structures of the cation shown, shifting only one electron pair in each step. See table 1 in the background. 1) there is only one real structure for each molecule or. Sometimes one dot structures is not enough to completely describe a molecule or an ion, sometimes you need two or more, and here's an example: Web draw the resonance structures of molecules or ions that exhibit delocalization. Be sure to include the formal charge on structures b and c. Be sure to include the formal charge on structures b and c. Use formal charges to determine.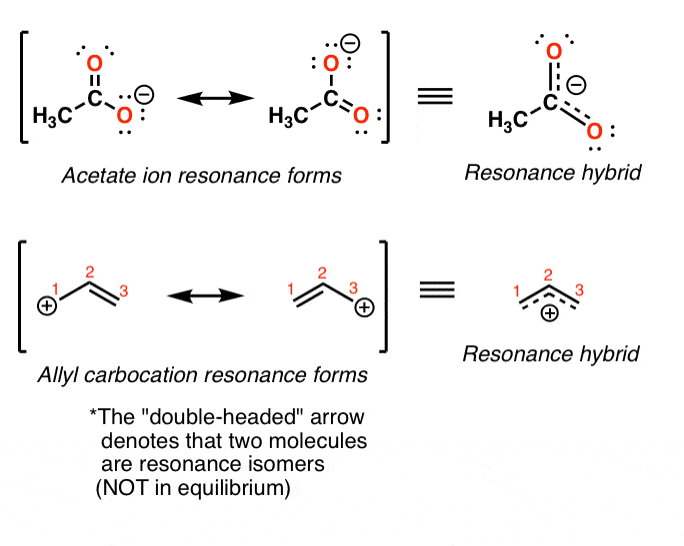
Resonance Structures 4 Rules On How To Evaluate Them, With Practice
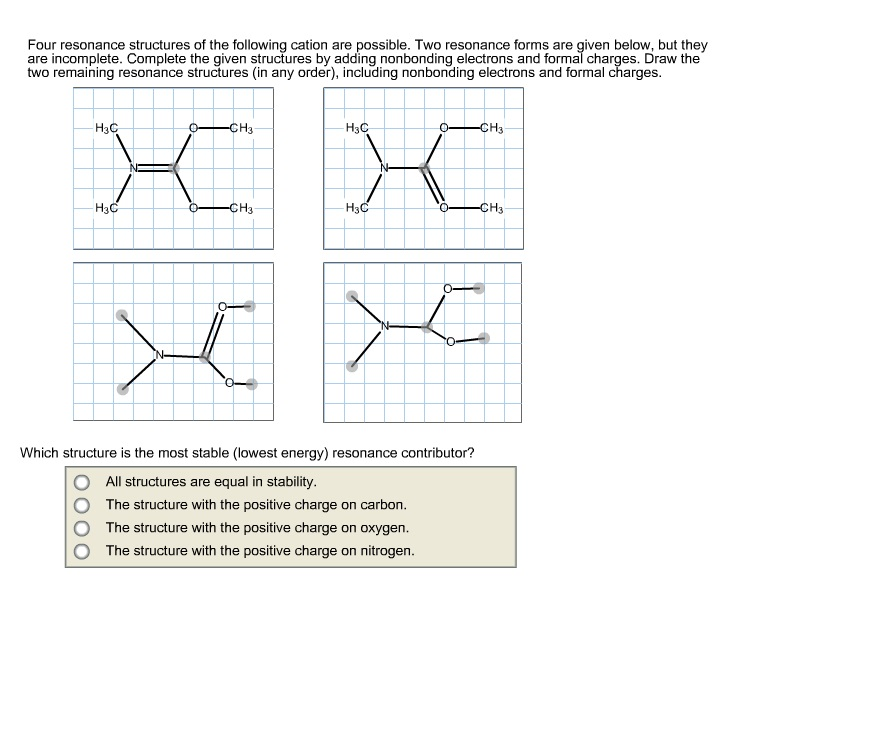
draw two resonance structures of the cation shown below
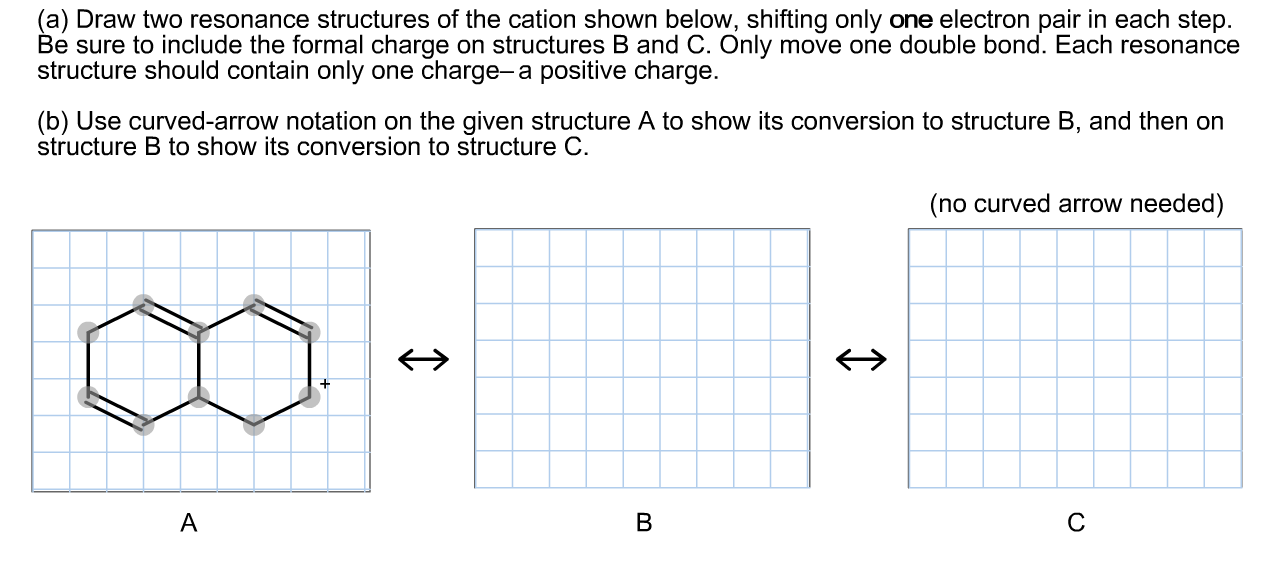
Solved (a) Draw two resonance structures of the cation shown
Resonance Is A Mental Exercise And Method.
Web Draw The Partially Expanded… | Bartleby.
(A) Draw Two Resonance Structures Of The Cation Shown, Shifting Only One Electron Pair In Each Step.
Determine The Relative Stability Of Resonance Structures Using A Set Of.
Related Post: