Draw The Resonance Contributors For
Draw The Resonance Contributors For - The general approach is described below: Follow the rules to determine the major resonance contributor. Because of the low hydrogen to carbon ratio in aromatic compounds (note that the h:c ratio in an alkane is >2), chemists expected their structural formulas would contain a large number of double or triple bonds. Include in your figure the appropriate curved arrows showing how you got from the given structure to your structure. The actual structure isn't part of the time one structure and part of the time another structure. Draw resonance contributors for each of the following species and rank them in order of decreasing contribution to the resonance hybrid. We just need a graphical tool to do it. Web when you draw resonance structures in your head, think about what that means for the hybrid, and how the resonance structures would contribute to the overall hybrid. Web it is useful to combine the resonance structures into a single structure called the resonance hybrid that describes the bonding of the molecule. Web why atoms react to form molecules? Web each lewis structure that contributes to the resonance hybrid is a resonance structure. Web here, we will focus on how to draw resonance structures (or resonance contributors) for organic chemistry species and how to compare the relative stabilities between the structures. Draw the resonance contributors for the phenolate ion. Part d draw the resonance contributors for draw all possible. Label each one as major or minor (the structure below is of a major contributor). We just need a graphical tool to do it. Web these two drawings are an example of what is referred to in organic chemistry as resonance contributors: Draw all possible resonance structures by copying the skeleton shown. Where there can be a double or triple. Two good lewis structures for benzene exist that differ only in their placement of double bonds. Resonance structures and major contributor. Include in your figure the appropriate curved arrows showing how you got from the given structure to your structure. Where there can be a double or triple bond, draw a dotted. Web these two drawings are an example of. Draw the important resonance contributors for the sigma complex shown here: Web more resonance contributors can be drawn in which negative charge is delocalized to three other atoms on the molecule. Resonance contributors and the resonance hybrid. Write the resonance structure (s) and identify the major contributor to the real molecule. 29 views 6 years ago fundamentals of organic chemistry. According to the resonance effect, the greater the number of resonance contributors, the greater the resonance stabilization effect, and the more stable the. For example, we can draw three possible contributors for formamide, hconh₂. Explain why your contributor is the major one. Include in your figure the appropriate curved arrows showing how you got from the given structure to your structure. Draw the resonance structure (s). Web why atoms react to form molecules? Draw resonance contributors for each of the following species and rank them in order of decreasing contribution to the resonance hybrid. If either structure were correct, benzene would consist of alternating long single bonds and short double bonds. Two good lewis structures for benzene exist that differ only in their placement of double bonds. The actual structure isn't part of the time one structure and part of the time another structure. Label each one as major or minor (the structure below is of a major contributor).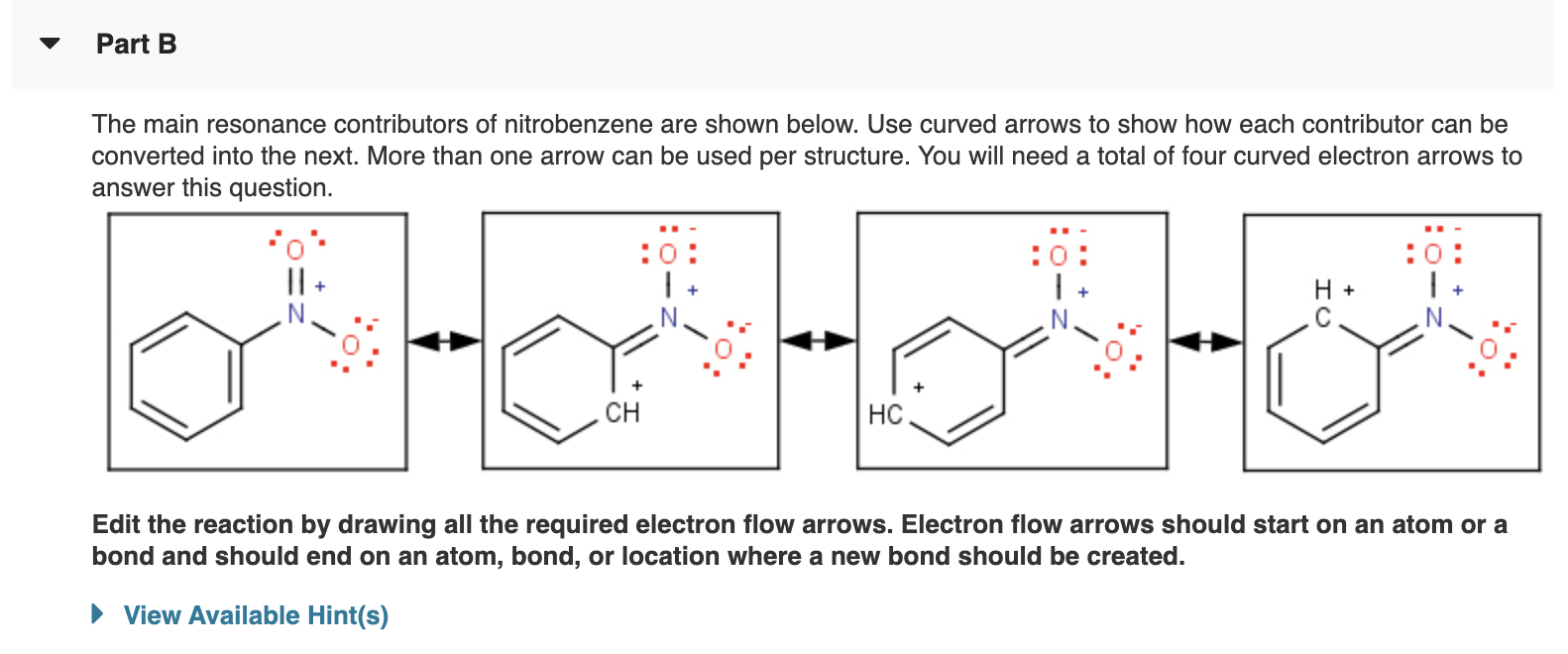
Solved Part B The main resonance contributors of

How to Draw Resonance Contributors MCC Organic Chemistry
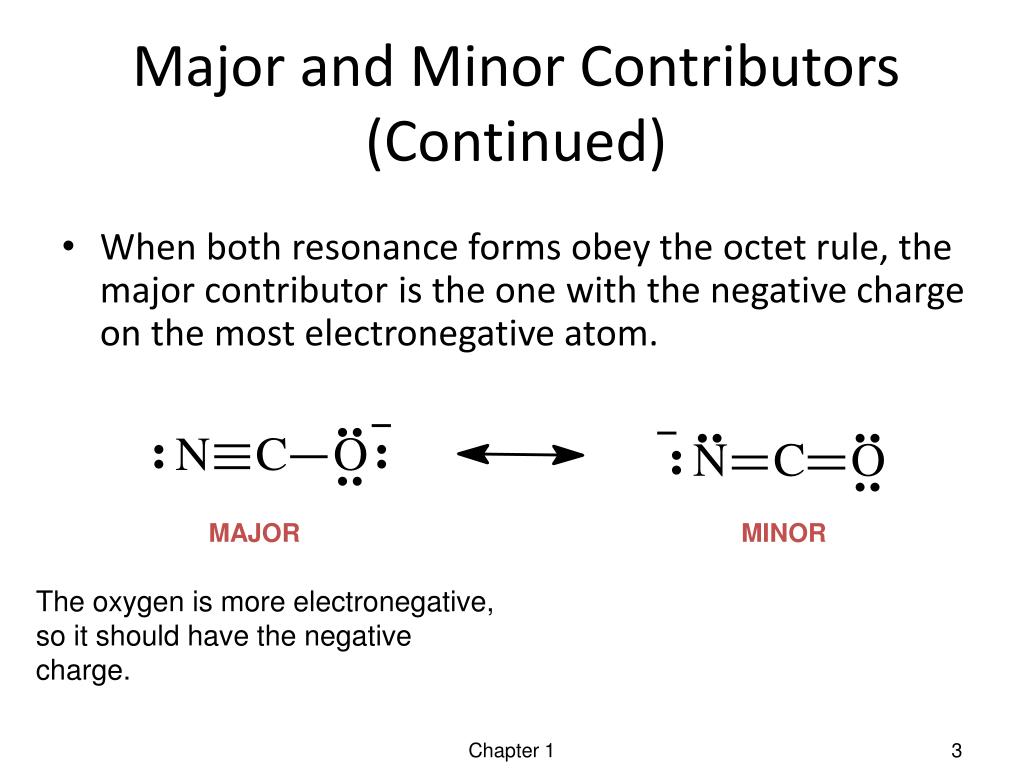
PPT Resonance Forms PowerPoint Presentation, free download ID1981144
Web Here, We Will Focus On How To Draw Resonance Structures (Or Resonance Contributors) For Organic Chemistry Species And How To Compare The Relative Stabilities Between The Structures.
Indicate Which Would Be The Major Contributor To The Resonance Hybrid.
Web Introducing Curved Arrows, A Tool For Showing The Movement Of Electrons Between Resonance Structures.
The Structure Is At All Times A Single Resonance Hybrid Of All The Structures.
Related Post: