Draw The Lewis Structure Of Ph3
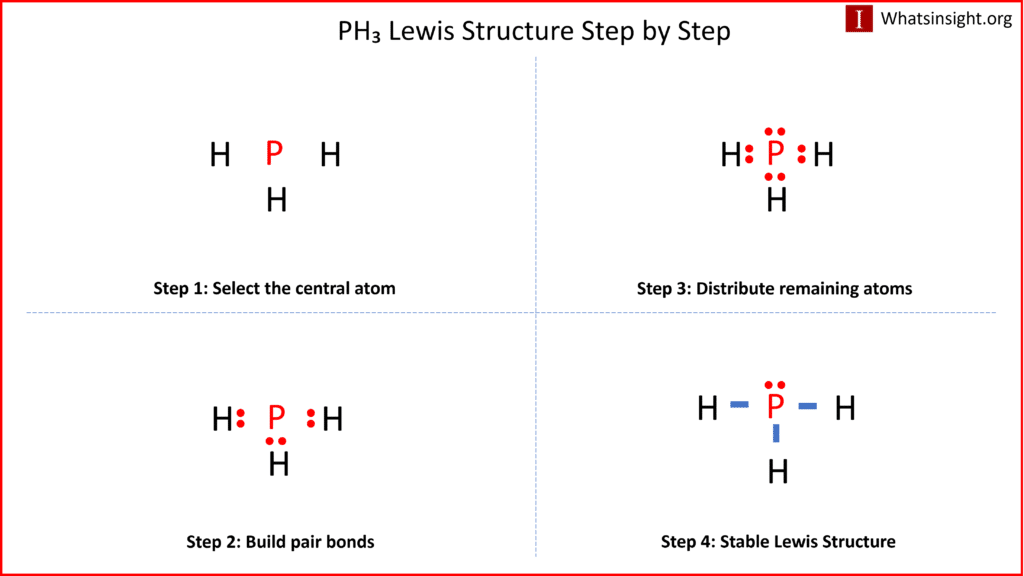
Draw The Lewis Structure Of Ph3 - 49k views 11 years ago chemistry lewis dot structures. Web lewis structure for ph3. Decide which atom is the central atom in the structure. Phosphorus (p) contributes 5 valence electrons, and each hydrogen (h) contributes 1. That will normally be the least electronegative atom (p). Drawings, hybridization, shape, charges, pair and detailed facts. Figure out how many electrons the molecule must have, based on the number of valence electrons in each. In this article, we are going to study ph3 lewis structure and various facts about it. Web lewis structure of ph3 contains three single bonds between the phosphorus (p) atom and each hydrogen (h) atom. Phosphorus, group 5, 5 valence electrons; By the end of this section, you will be able to: Phosphosus has an electron configuration of [n e]2s22p3. Include lone pairs of electrons and hydrogen atoms. Phosphorus (p) contributes 5 valence electrons, and each hydrogen (h) contributes 1. May 7, 2022 by sania jakati. Here are the steps that i follow when drawing a lewis structure. Here’s the best way to solve it. Phosphosus has an electron configuration of [n e]2s22p3. * each hydrogen brings 1 electron * this allows for three single bonds and one lone pair of. The electron the steric number is pair geometry is molecular geometry is | and the. Ph3 or phosphine is a compound of phosphorus that is classified under pnictogen hydride. Let's do the ph3 lewis structure. Draw lewis structures depicting the bonding in simple molecules. 92% (12 ratings) share share. We draw lewis structures to predict: Web lewis structure is the pictorial representation of the arrangement of atoms and valence electrons in the molecule. Draw lewis structures depicting the bonding in simple molecules. For the ph3 structure use the periodic table to find the total number of. Figure out how many electrons the molecule must have, based on the number of valence electrons in each. The molecular formula of phosphene is ph3 which indicates the compound has one phosphorous atom bonding with three hydrogen atoms. Draw the lewis structure of ph3. B) draw a lewis structure for so42∗ in which all atoms obey the octet rule. That will normally be the least electronegative atom (p). Draw the lewis structure of ph3. Phosphorus, group 5, 5 valence electrons; (a) how many hydrogen atoms are in the molecule? Thus far in this chapter, we have discussed the various types of bonds that form between atoms and/or ions. Drawings, hybridization, shape, charges, pair and detailed facts. The phosphorus atom has one lone pair. Web in the ph 3 lewis structure, there are three single bonds around the phosphorus atom, with three hydrogen atoms attached to it, and on the phosphorus atom, there is one lone pair. To add bonds connect atoms with a line draw the molecule by placing atoms on the grid and connecting them with bonds.
Ph3 Estructura De Lewis Estudiar
PH3 Lewis Structure in four simple steps What's Insight

Ph3 Lewis Structure Shape
Phosphorus (P) Contributes 5 Valence Electrons, And Each Hydrogen (H) Contributes 1.
Identify The Number Of Valence Electrons For Each Atom In The Molecule.
* Phosphorus Requires A Full Octet Of Electrons, And Brings 5 With It.
In This Tutorial We Will Learn How To Draw The Lewis Structure Of Ph 3 And Determining The Shape And Molecular Geometry Of The Molecule.
Related Post: