Draw The Lewis Structure Of H2S
Draw The Lewis Structure Of H2S - It is composed of two hydrogen atoms and one sulfur atom, and is important in various industrial processes and biochemical reactions. In this compound, both the hydrogen atoms require one electron to make the covalent bond with sulfur. Web the lewis structure of h2s is as below. 40k views 2 years ago lewis structures. Determine the number of valence electrons: See the big list of lewis structures. Sum the valence electrons of each atom: Electrons are shown as dots or for bonding electrons as a line between the two atoms. Sulfur needs eight electrons to fulfill the requirements for octet rule. In a water molecule, an oxygen atom forms two bonds, one to each hydrogen atom. See the big list of lewis structures. By the end of this section, you will be able to: Hydrogen sulfide h 2 s is a gas with a foul smell, often described as being similar to rotten eggs. Web to properly draw the h 2 s lewis structure, follow these steps: In a water molecule, an oxygen atom forms two. Write lewis symbols for neutral atoms and ions. Valence electrons in hydrogen (h): The number of dots equals the number of valence electrons in the atom. Added jun 9, 2014 by webtester in chemistry. Web a lewis electron dot diagram (or electron dot diagram, or a lewis diagram, or a lewis structure) is a representation of the valence electrons of. Web to properly draw the h 2 s lewis structure, follow these steps: See the big list of lewis structures. First and foremost it is important to determine how many valence electrons are present in the compound. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Draw the lewis dot structure. By the end of this section, you will be able to: The example is for the nitrate ion. Hydrogen valence electron = 1. 2 electrons (1 electron per atom) x 2 atoms = 2 electrons See the big list of lewis structures. Bond order, bond distance, and bond energy. Interested in an albert school license? Thus far in this chapter, we have discussed the various types of bonds that form between atoms and/or ions. It is composed of two hydrogen atoms and one sulfur atom, and is important in various industrial processes and biochemical reactions. Web atoms can form more than one bond. Let's do the lewis structure for h2s: Hydrogen sulfide h 2 s is a gas with a foul smell, often described as being similar to rotten eggs. Concept of number of total valence electrons of sulfur and hydrogen atoms are used to draw lewis structure of h 2 s. A lewis dot structure can be made for a single atom, a covalent compound, or a polyatomic ion. The number of dots equals the number of valence electrons in the atom. #1 draw a rough sketch of the structure.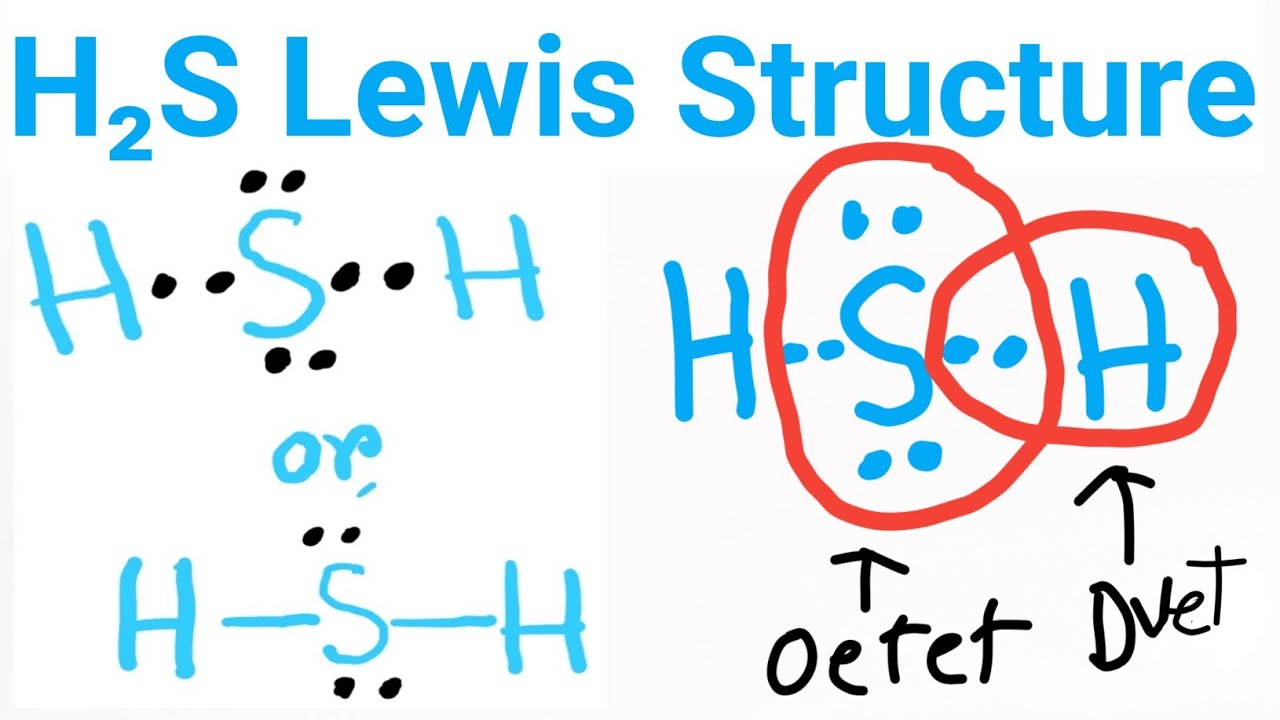
H2S Lewis Structure Lewis Dot Structure for H2S Hydrogen sulfide
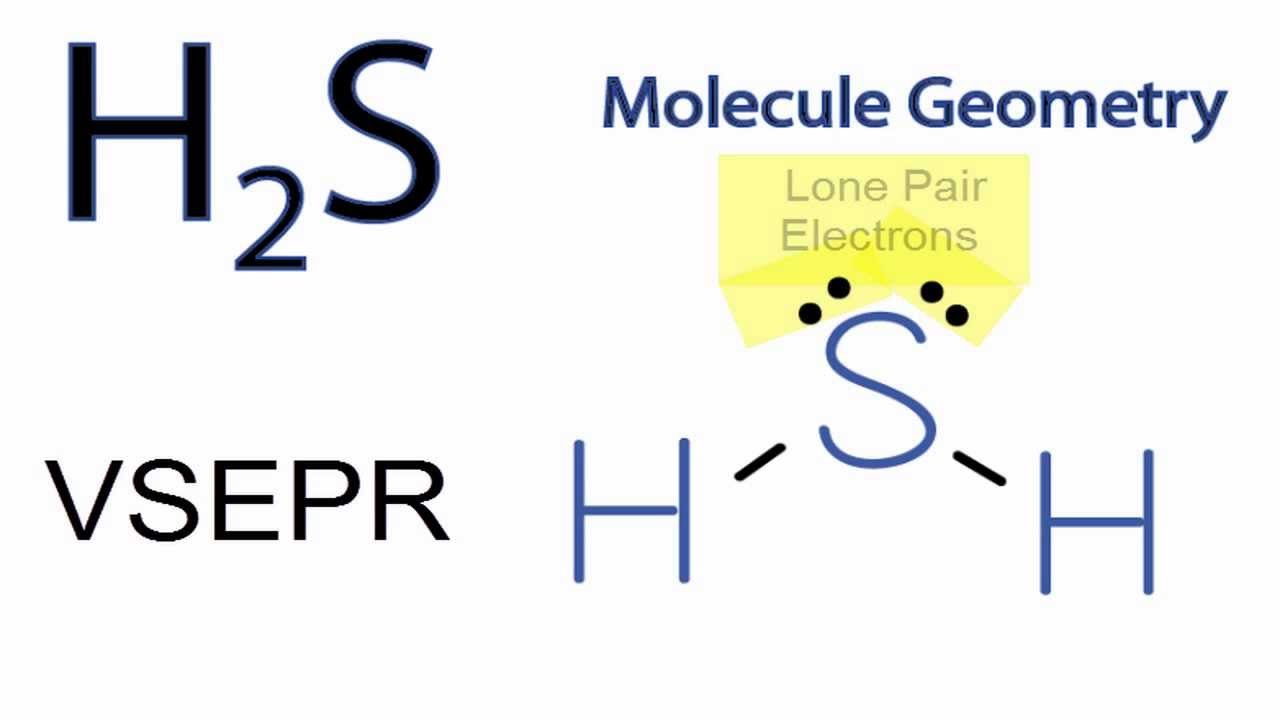
H2S Lewis Structure, Molecular Geometry, Hybridization and Polarity
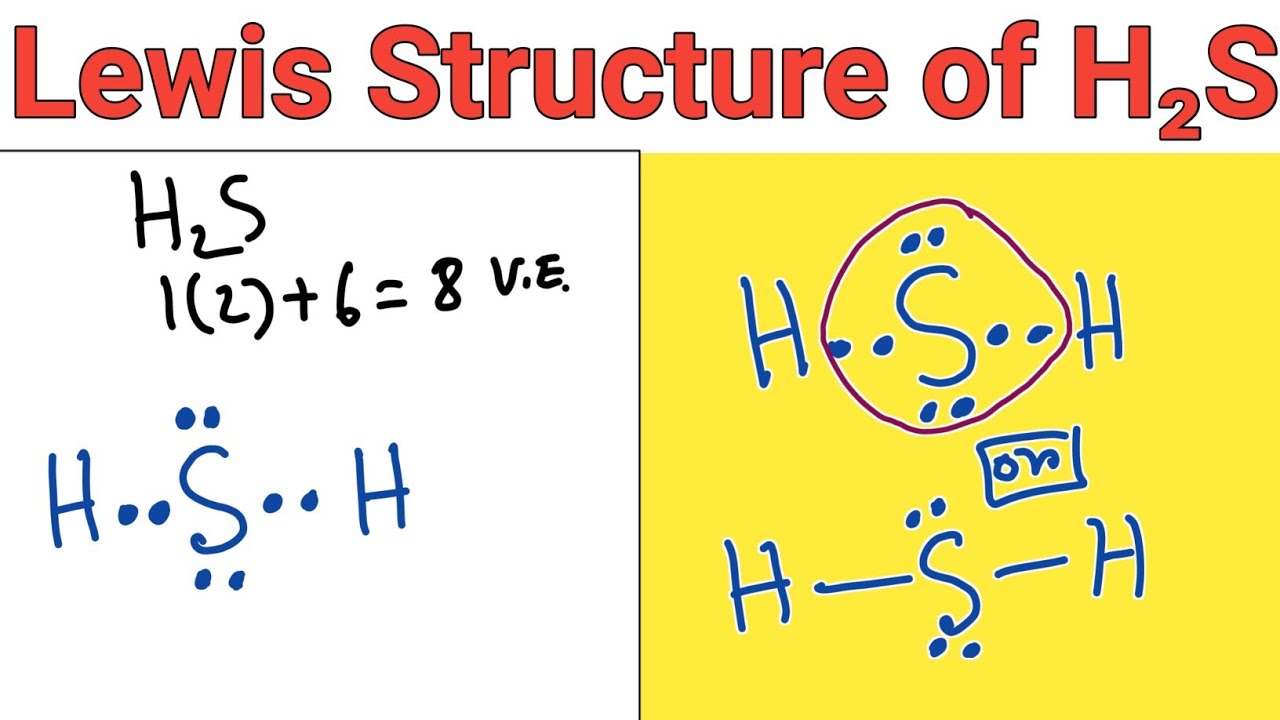
Lewis structure of H2S (Hydrogen sulphide) YouTube
First, Determine The Total Number Of Valence Electrons.
#3 Indicate Formal Charges On The Atoms, If Necessary.
Valence Electrons In Hydrogen (H):
Web Draw Lewis Structures Depicting The Bonding In Simple Molecules.
Related Post: