Draw The Lewis Structure Of Ch4
Draw The Lewis Structure Of Ch4 - There are several possible lewis. Remember that hydrogen atoms always go on the outside of a lewis structure and that they only need two valence electrons for a full outer shell. We're going to draw the dot structure for ch 4, carbon tetrahydride, also called methane. 45k views 10 years ago. How to draw the lewis structure of ch4 (methane) chemistnate. Answer to solved draw the lewis structure for ch4. Assign formal charge to an atom in a dot structure. Methane is one of the simple organic molecules, given its straightforward structure. There are no lone pairs on both carbon a. Electrons are shown as dots or for bonding electrons as a line between the two atoms. Determine the total number of valence electrons in the molecule. How to draw the lewis structure of ch4 (methane) chemistnate. Ch4 lewis structure, hybridization, molecular geometry, bond angle and shape. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. View the full answer step 2. Assess the stability of a structure by considering formal charges of atoms. Remember that hydrogen atoms always go on the outside of a lewis structure and that they only need two valence electrons for a full outer shell. Answer to solved draw the lewis structure for ch4. The diagram is also called a lewis dot diagram, lewis dot formula, or. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. 69k views 12 years ago every video. Figure out how many electrons the molecule must have, based on the number of valence electrons in each. Draw the lewis dot structure of a given molecule or ion. Web lewis. Methane is a simple molecule, consisting of one carbon atom bonded to four hydrogen atoms. For the ch4 structure use the periodic table to find the total number of. Draw resonance structures of some molecules. For very simple molecules and molecular ions, we can write the lewis structures by merely pairing up the unpaired electrons on. Electrons are shown as dots or for bonding electrons as a line between the two atoms. How can i draw a lewis structure of a compound? Web lewis dot structures. Figure out how many electrons the molecule must have, based on the number of valence electrons in each. Determine the total number of valence electrons in the molecule. Web drawing the lewis structure for ch 4 (named methane) requires only single bonds. Moreover, as there exist sigma bonds only and one 2s and three 2p orbitals of the carbon produce four new hybrid orbitals, the hybridization of ch4 is sp3. This widget gets the lewis structure of chemical compounds. Answer to solved draw the lewis structure for ch4. Ch4 lewis structure, hybridization, molecular geometry, bond angle and shape. For the ch4 structure use the periodic. Here’s how you can draw its lewis structure: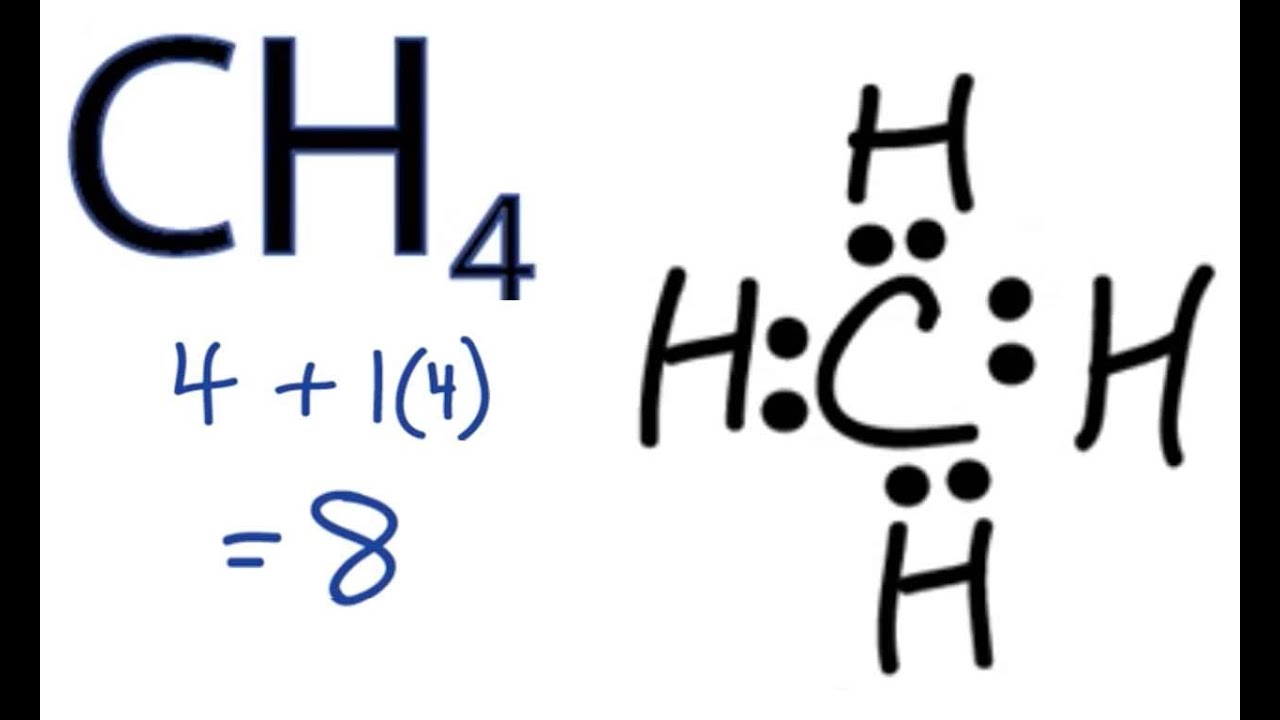
CH4 Lewis Structure How to Draw the Dot Structure for CH4 (Methane

In this video we are going to learn about the Lewis structure of CH4

Electron Dot Diagram For Methane
How To Draw The Lewis Structure Of Ch4 (Methane) Chemistnate.
There Are Several Possible Lewis.
Remember That Hydrogen Atoms Always Go On The Outside Of A Lewis Structure And That They Only Need Two Valence Electrons For A Full Outer Shell.
The Carbon Atom (C) Is At The Center And It Is Surrounded By 4 Hydrogen Atoms (H).
Related Post: