Draw The Lewis Structure For Xef2
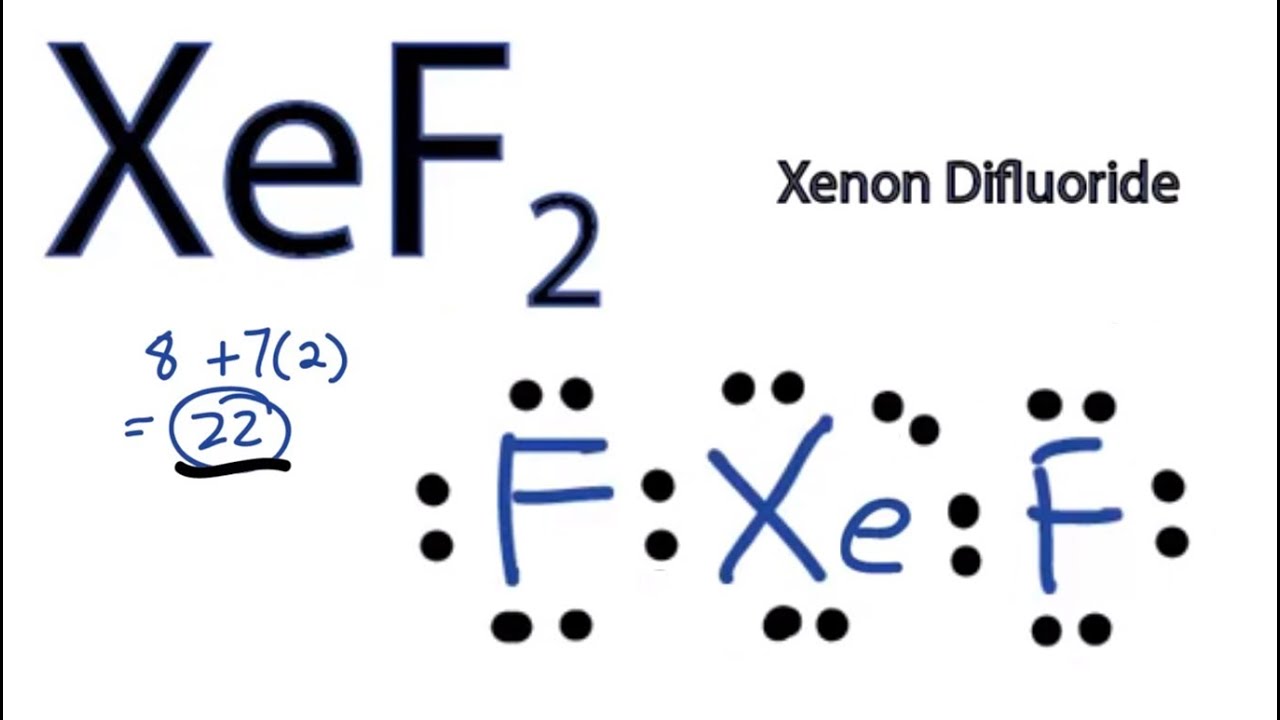
Draw The Lewis Structure For Xef2 - #1 first draw a rough sketch. Web xef2 lewis structure is the abbreviation of xenon difluoride. Web in some molecules, the central atom exceeds the octet rule (is surrounded by more than eight electrons). What is the electronic geometry of this molecule (look at. Find the total valence electrons in xef2 molecule. 65k views 10 years ago. Web to create the lewis structure of xef2, we follow these steps: Web chemistry learning made easy.this tutorial will help you deal with the lewis structure and moleculargeometry for xenon difluoride (xef2). In order to find the total valence electrons in xef2. Sp4 a draw a lewis structure for the ammonia molecule (nh3) and complete the following statement: Find the total valence electrons in xef2 molecule. What is the electronic geometry of this molecule (look at. Web the lewis structure of xef2 depicts the arrangement of atoms and valence electrons in a molecule of xenon difluoride. Steps of drawing xef2 lewis structure. Web use these steps to correctly draw the xef 2 lewis structure: First, determine the total number of valence electrons in the. Sp4 a draw a lewis structure for the ammonia molecule (nh3) and complete the following statement: #3 calculate and mark formal charges on. Web the lewis structure of xef2 depicts the arrangement of atoms and valence electrons in a molecule of xenon difluoride. In order to find the total valence. There are two single bonds between the. Web the lewis structure of xef2 depicts the arrangement of atoms and valence electrons in a molecule of xenon difluoride. It shows xenon (xe) as the central atom bonded to two. Steps of drawing xef2 lewis structure. Web use these steps to correctly draw the xef 2 lewis structure: First, determine the total number of valence electrons in the. In order to find the total valence electrons in xef2. Web the lewis structure of xef2 depicts the arrangement of atoms and valence electrons in a molecule of xenon difluoride. Steps of drawing xef2 lewis structure. There are two single bonds between the. What is the electronic geometry of this molecule (look at. The lewis structure for xef 2 requires you to place more than 8 valence electrons on xe. 65k views 10 years ago. Draw the lewis structure for xef2 a) how many groups (atoms and lone pairs) surround the central oxygen? For the xef2 lewis structure we first count the valence electrons for the xef2 molecule using the periodic table. #2 mark lone pairs on the atoms. #3 calculate and mark formal charges on. The xef2 lewis structure consists of a central xenon atom (xe) and two external fluorine atoms (f). It is one of those rare compounds which involve noble gases despite their strong stability. The lewis structure of a given chemical compound is crucial for knowing all the physical properties and chemical. Web xef2 lewis structure is the abbreviation of xenon difluoride.![Molecular geometry of XeF2 [with video and free study guide]](https://aceorganicchem.com/chemistry/wp-content/uploads/2023/06/XeF2-lewis.jpg)
Molecular geometry of XeF2 [with video and free study guide]

Xef2 Lewis Structure Resonance Draw Easy
XeF2 Lewis Structure How to Draw the Lewis Structure for XeF2 YouTube
It Shows Xenon (Xe) As The Central Atom Bonded To Two.
Drawing The Lewis Structure For Xef2.
See An Example Of A Molecule That Violates The Octet Rule (Xef₂) And.
Hydrogen (H) Only Needs Two Valence.
Related Post: