Draw The Lewis Structure For The Nitronium Ion
Draw The Lewis Structure For The Nitronium Ion - Write the lewis structure of the nitrite ion, n o2−. Web how to draw lewis structure for no2+ nitronium ionlewis structure: Draw a suitable lewis dot structure for. 100% (1 rating) share share. Draw one structure per sketcher box, and separate any added sketcher boxes with the ↔ symbol. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Do not forget to subscribe! There are 2 steps to solve this one. Move electrons so all atoms (esp. The nitrogen atom forms a double bond with one of the oxygen atoms and a coordinate covalent bond with the other oxygen atom, resulting in a bond order of 2. Web to draw the no2+ lewis structure, follow these steps: A lewis structure is a way to show how atoms share electrons when they form a molecule. Interested in an albert school license? Lewis structures depict the conf. Identify the central atom, which is nitrogen (n). Show all of the valence electrons. A lewis structure is a way to show how atoms share electrons when they form a molecule. This cation no2 plus is a strong electrophile.… It is created by removal of an electron from the nitrogen dioxide molecule. Common ion effect (0) precipitation: This cation no2 plus is a strong electrophile.… Web the total number of valence electrons available for drawing the nitronium (no2+) ion lewis structure is 16. This problem has been solved! 1k views 2 years ago. Part a.) draw lewis structure (s) showing all possible equivalent resonance forms for the nitronium ion ( no2+ ). Write the lewis structure of the nitrite ion, n o2−. Draw one structure per sketcher box, and separate any added sketcher boxes with the ↔ symbol. Ksp vs q (0) selective precipitation (0) complex ions: Draw lewis structures for c2− 2 ions. For this reason it has a similar vibrational spectrum to carbon dioxide. Web the lewis electron structure for the nh 4 + ion is as follows: To draw lewis structures for molecules and polyatomic ions with one central atom. Draw the lewis structures for the following molecules and ions : It is created by removal of an electron from the nitrogen dioxide molecule. Using the periodic table to draw lewis dot. Web h2so4 + hno3 → hso− 4 + [no2]+ + h2o. Web put one electron pair in each bond 4. View the full answer step 2. Show all of the valence electrons. Using equation 4.4.1 , the formal charge on the nitrogen atom is therefore Distribute the remaining electrons around each oxygen atom, following the octet rule.
How to draw the Lewis structure of NO2 + (Nitronium ion) YouTube
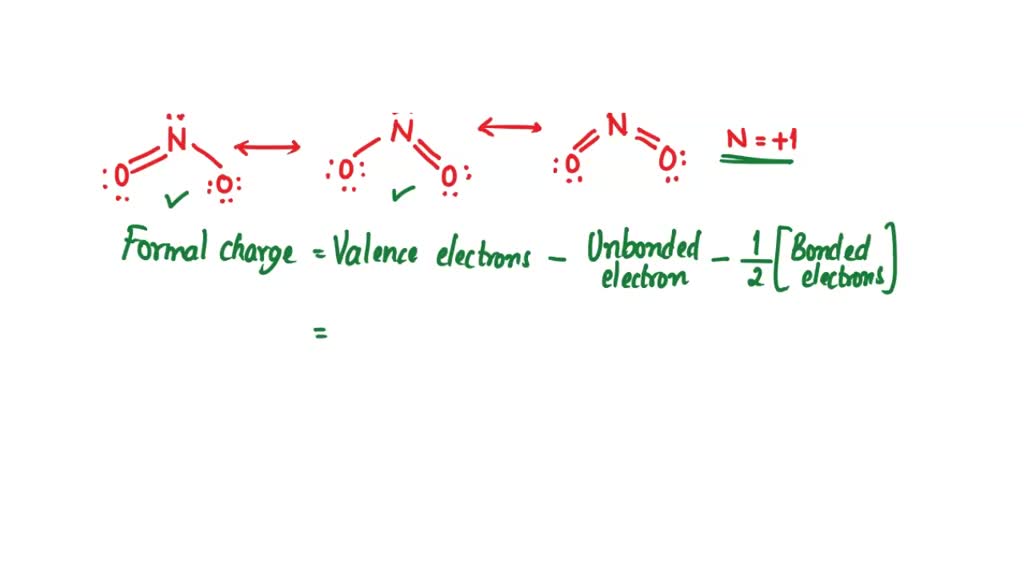
SOLVED One Lewis structure for the nitronium ion contains two double

Lewis structure of Nitronium (NO2+) ion. How to draw Lewis structure
Lewis Structures Depict The Conf.
In The Space Below, Draw A Reasonable Lewis Electron Dot Structure For The Nitronium Ion, No2+.
Do Not Show Any Ion Charges In Your Drawings.
Web Lewis Structures Are Drawn By A Series Of Dots, Lines, And Atomic Symbols And Provide A Structure For The Way That The Atom Or Molecule Is Arranged.
Related Post: