Draw The Lewis Structure For The Hcn
Draw The Lewis Structure For The Hcn - Web draw lewis structures depicting the bonding in simple molecules. Web what is the lewis structure of hcn? The lewis structure of hcn shows the arrangement of atoms and electrons in the molecule. Thus far in this chapter, we have discussed the various types of bonds that form between atoms and/or ions. Be sure that you don't use more than the ten valence electrons available. Find the total valence electrons in hcn molecule. Also, helium is shown in group 8a, but it only has two valence electrons. According to this theory, bond will form when. For hcn, hydrogen has one valence electron, carbon has four valence electrons, and nitrogen has five valence electrons, making a total of ten valence electrons. #5 repeat step 4 if needed, until all charges are minimized, to get a stable. The lewis structure of hcn shows that the carbon atom is the central atom and is covalently bonded to both hydrogen and nitrogen atoms. And does it exhibit resonance? According to this theory, bond will form when. Web though we have seen the lewis structure of hcn we need to see how the 3d representation of the compound looks like.. The hcn molecule consists of three atoms: Web draw lewis structures depicting the bonding in simple molecules. Draw the lewis structure of hcn and then determine its electron domain and molecular geometries. #3 calculate and mark formal charges on the atoms, if required. According to this theory, bond will form when. Find the total valence electrons in hcn molecule. How to draw the lewis structure for hcn. Web draw the lewis dot structure of hydrogen cyanide (hcn) molecule. Use these steps to correctly draw the hcn lewis structure: Thus far in this chapter, we have discussed the various types of bonds that form between atoms and/or ions. The lewis structure of hcn shows the arrangement of atoms and electrons in the molecule. Here is a little flow chart of how we are going to do this: For the hcn lewis structure, calculate the total number of valence electrons. Here, the given molecule is hcn. Put least electronegative atom in centre3. Also, helium is shown in group 8a, but it only has two valence electrons. #2 mark lone pairs on the atoms. Web to draw the lewis structure of an atom, write the symbol of the atom and draw dots around it to represent the valence electrons. In order to draw the lewis structure of hcn, first of all you have to find the total number of valence electrons present in the hcn molecule. #3 calculate and mark formal charges on the atoms, if required. Hcn is a linear molecule, since it has only two electron groups and there are no lone pairs on the central carbon atom. 284k views 10 years ago lewis structures practice problems with answers. Put the least electronegative atom c in the middle with h and cl on either side. Web drawing lewis structures. Web learn to draw the lewis structure of hcn & understand molecular geometry, shape, & polarity about the same by reading this article. And to find that we need to find the molecular geometry of the compound.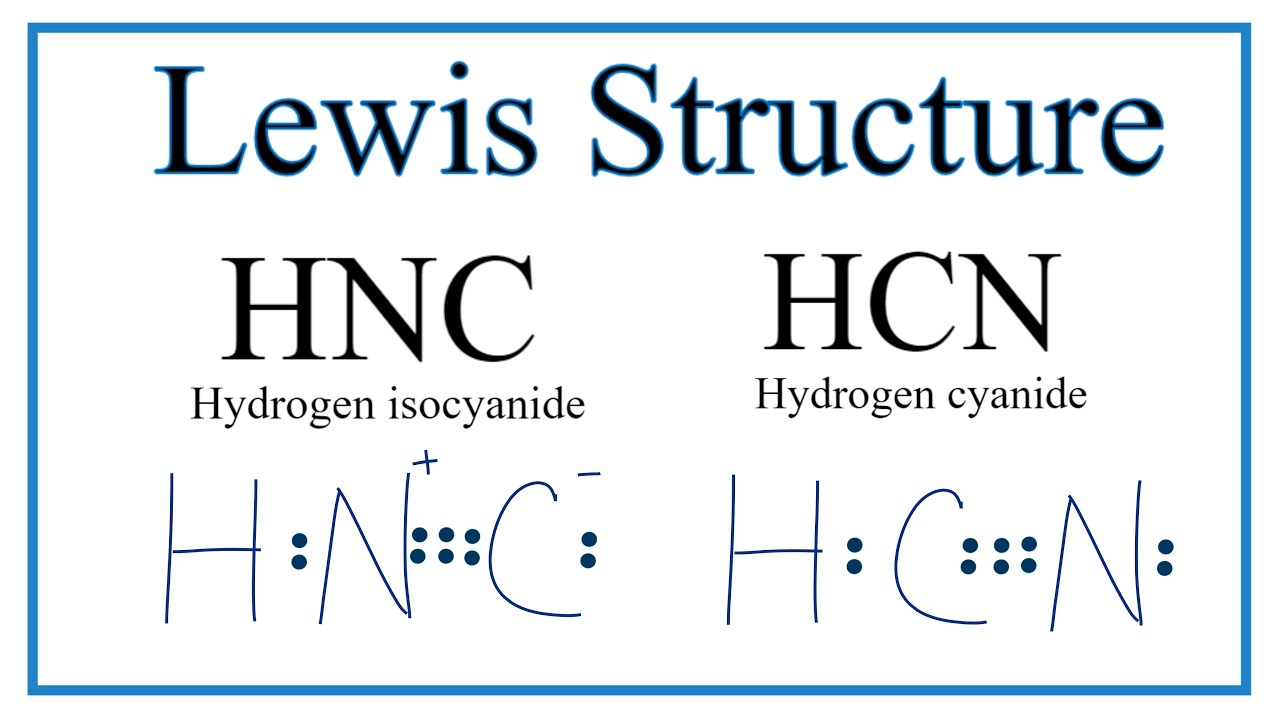
Hcn Lewis Structure Bonds Draw Easy

HCN Lewis StructureHydrogen Cyanide (HCN) Lewis Dot StructureDraw
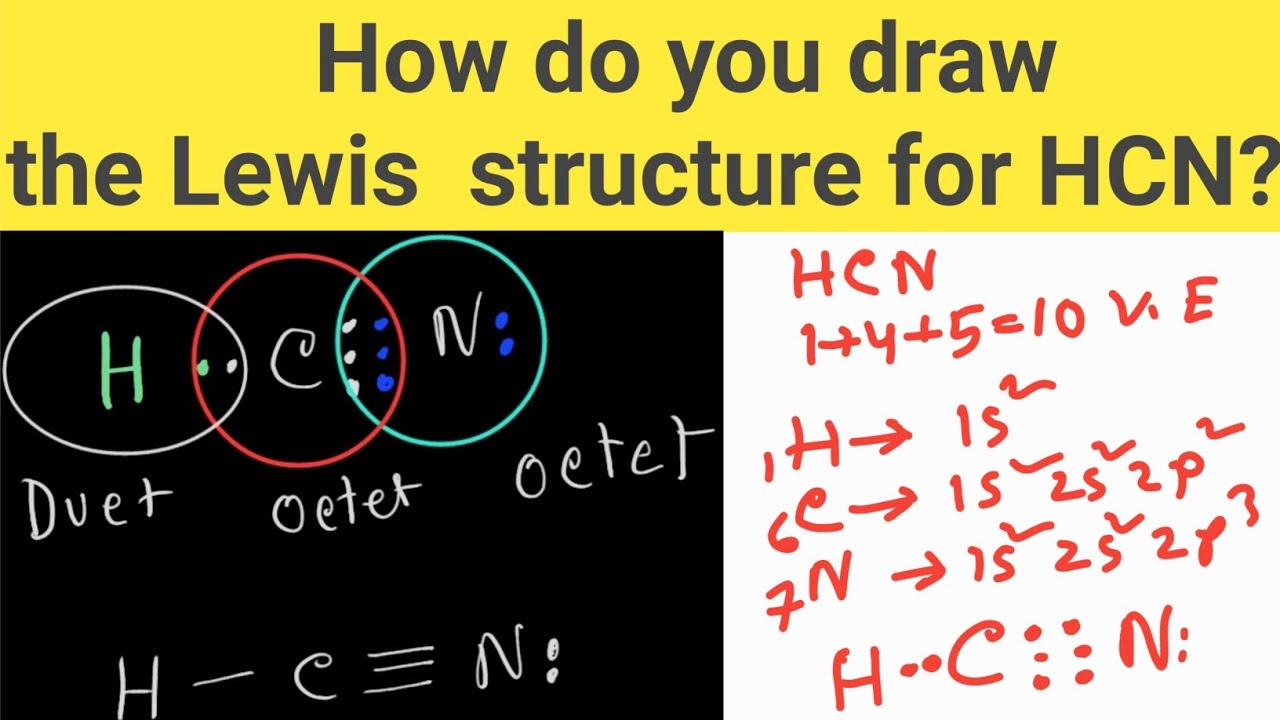
How do you draw the Lewis structure of HCN (hydrogen cyanide)? HCN
Count The Valence Electrons You Can Use.
Put One Electron Pair In Each Bond4.
Here's How To Do It.
There Are 2 Steps To Solve This.
Related Post: