Draw The Lewis Structure For The Conjugate Acid Of Ammonia
Draw The Lewis Structure For The Conjugate Acid Of Ammonia - Web the resulting lewis structure for the conjugate acid of ammonia (nh4+) is: Ammonia (nh3) can behave as both an acid and a base. The formula for ammonia is nh3. Ammonia (nh3) can behave as both and acid and a base. Draw the lewis structure for the conjugate acid of ammonia. Ammonia is both a brønsted and a lewis base, owing to the unshared electron pair on the nitrogen. Craig beals shows how to draw the lewis structure for ammonia. Web draw the lewis structure for the conjugate acid of ammonia. This is a clip from the. The lewis structure for the conjugate acid of ammonia (nh4+) will have the nitrogen atom bonded to four hydrogen atoms, and a positive charge on the. This is a clip from the. Draw the lewis structure for the conjugate base of ammonia. Ammonia (nh3) can behave as both an acid and a base. Web this chemistry video tutorial explains how to draw the lewis structure of nh3 also known as ammonia.how to draw lewis structures: Craig beals shows how to draw the lewis structure for ammonia. Count the total number of valence electrons. Web lewis structure of ammonia—a nitrogen with a lone pair of electrons that is also bound to 3 hydrogens—plus the lewis structure of hydrochloric acid forms ammonium. Draw the lewis structure for the conjugate acid of ammonia. On the other hand, the conjugate base of ammonia is formed when ammonia donates. The stronger. Lewis structure of nh3 can be drawn by using valence electrons of nitrogen and. On the other hand, the conjugate base of ammonia is formed when ammonia donates. Draw the lewis structure for the conjugate acid of ammonia. Web draw the lewis structure for the conjugate acid of ammonia. Web chemistry questions and answers. Lewis structure of nh3 can be drawn by using valence electrons of nitrogen and. The formula for ammonia is nh3. Draw the lewis structure for the conjugate acid of ammonia. This is a clip from the. Web this chemistry video tutorial explains how to draw the lewis structure of nh3 also known as ammonia.how to draw lewis structures: Craig beals shows how to draw the lewis structure for ammonia. Web draw the lewis structure for the conjugate acid of ammonia. Draw the lewis structure for the conjugate base of ammonia. Web note that the conjugate base is also the adduct. The simplest amino acid is glycine, h 2 nch 2 co 2 h. Ammonia (nh3) can behave as both and acid and a base. The stronger an acid, the weaker. Web draw the lewis structure for the conjugate acid of ammonia. Count the total number of valence electrons. Select draw templates more erase draw the lewis structure for the conjugate base of ammonia. The lewis structure for the conjugate acid of ammonia (nh4+) will have the nitrogen atom bonded to four hydrogen atoms, and a positive charge on the.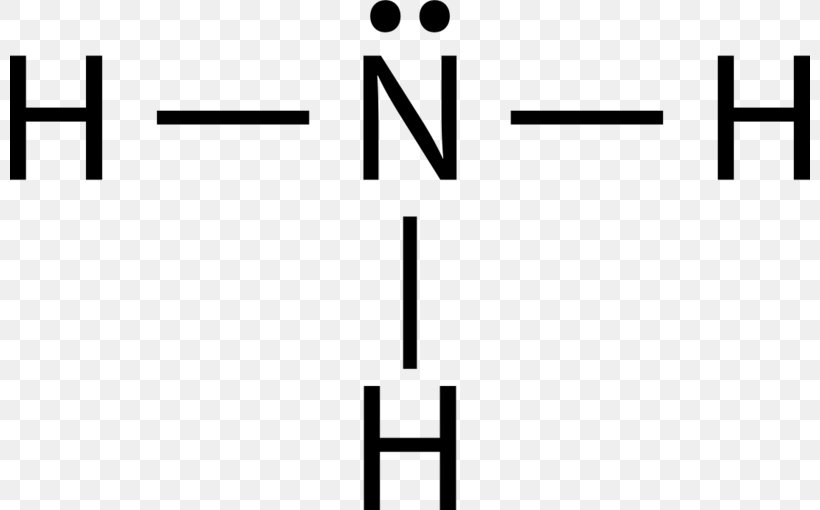
How To Draw The Lewis Structure For Ammonia Science E vrogue.co

Молекула аммиака рисунок 83 фото

Conjugate Acid of NH3 YouTube
Ammonium, N H + 4.
(Ii) The Conjugate Acid Of Ammonia Is The Ammonium Ion, Nh4 +.
Web (I) Draw The Lewis Structure Of Ammonia And State The Shape Of The Molecule And Its Bond Angles.
Web Chemistry Questions And Answers.
Related Post: