Draw The Lewis Structure For The Azide Ion
Draw The Lewis Structure For The Azide Ion - Web lewis structures are visual representations of the bonds between atoms and illustrate the lone pairs of electrons in molecules. Lewis structures show all of the valence electrons in an atom or molecule. Web to draw lewis structures for molecules and polyatomic ions with one central atom. When decomposed it rapidly forms nitrogen gas. Web draw the lewis structure for the azide (n3) ion. Draw the lewis structure of azide (n3−) and then determine the hybridization of the central atom. This problem has been solved! A video explanation of how to draw the lewis dot structure for the azide ion, along with information about the compound including formal charges, polarity, h. This makes a total of 16 electrons overall. Azide does have resonance structures. This problem has been solved! Nitrogen ordinarily has five electrons in its valence; This makes a total of 16 electrons overall. The azide ion is a conjugate base of hydrazoic acid (hn3). You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Draw the lewis structure for the most stable azide ion, n3. To get the lewis structure we have to count the number of valence electrons in azide ion. And then we have this negative sign up here so we're going to add an additional electron, for a total of 16 valence electrons. They can also be called lewis dot diagrams. Web to draw lewis structures for molecules and polyatomic ions with one central atom. I also go over hybridization, shape and bond angles. Azide does have resonance structures. Next lets draw the basic framework of the molecule: Web the lewis structure of the azide ion is: This makes a total of 16 electrons overall. This problem has been solved! Azide does have resonance structures. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. The central n atom carries a negative charge. Web the lewis structure of the azide ion is: (valence electrons are the number of electrons present in the outermost shell of an atom). Next lets draw the basic framework of the molecule: The valence electrons are the electrons in the. It has 5 valence electrons. Steps of drawing the lewis structure of. Web 70 more lewis dot structures. They can also be called lewis dot diagrams and are used as a simple way to show the configuration of atoms within a molecule. Web lewis structures are visual representations of the bonds between atoms and illustrate the lone pairs of electrons in molecules. Web to draw lewis structures for molecules and polyatomic ions with one central atom. Nitrogen ordinarily has five electrons in its valence;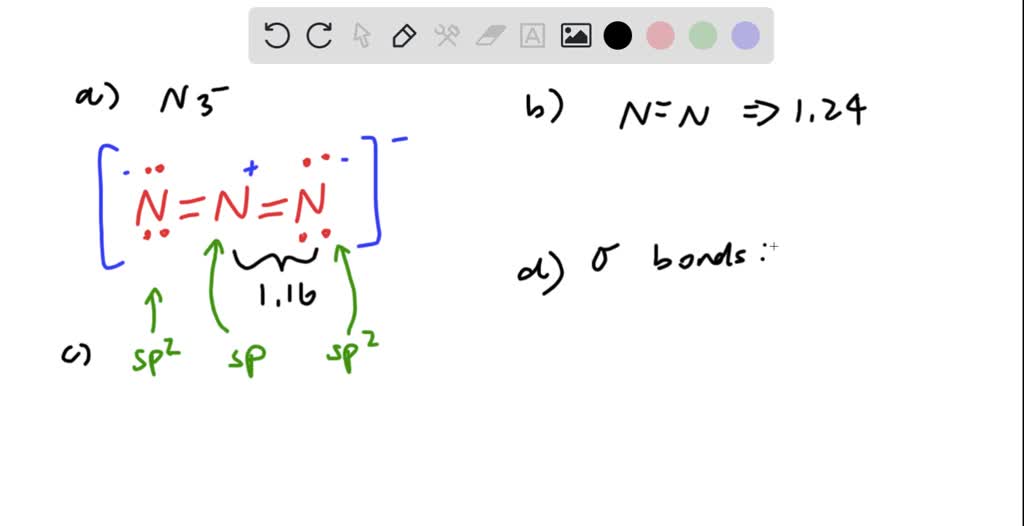
SOLVEDThe azide ion, N3^, is linear with two NN bonds of equal

How many resonance structures does N3 have?
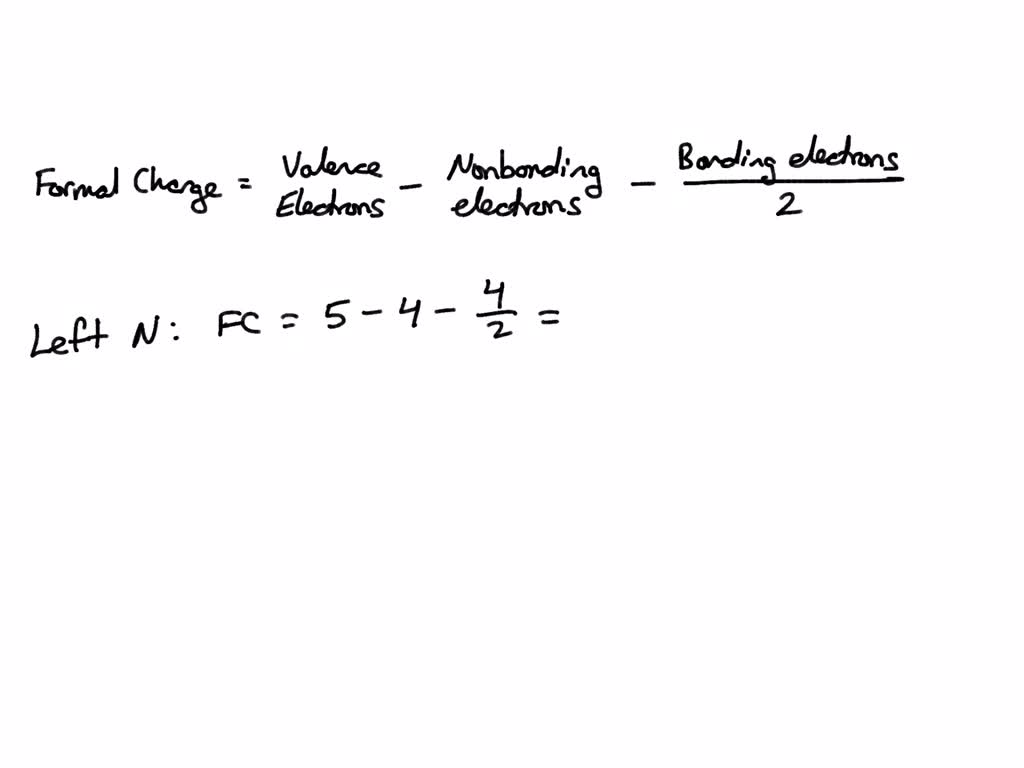
SOLVED Draw the Lewis structure of the azide ion, N3, and calculate
Select Draw Rings More N.
And Then We Have This Negative Sign Up Here So We're Going To Add An Additional Electron, For A Total Of 16 Valence Electrons.
Again Azide Ion Carries One Negative Charge.
The Ion Is Made Up Of Three Nitrogen Atoms.
Related Post: