Draw The Lewis Structure For The Ammonium Ion
Draw The Lewis Structure For The Ammonium Ion - The following procedure can be used to construct lewis electron structures for more complex molecules and. The following procedure can be used to construct lewis electron structures for more complex molecules and ions. Web they follow the duet rule (2 electrons). Name of molecule ammonium ion; Show the calculation of the formal charge on nitrogen and. Now that we have drawn the nh4+ lewis structure, let’s discuss what it means. Web the nh4+ lewis structure is a representation of the ammonium ion, which consists of four hydrogen atoms bonded to a central nitrogen atom. Web the ion is isoelectronic with methane and borohydride and has a tetrahedral structure. The following procedure can be used to construct lewis electron structures for more. This problem has been solved! Web let's do the lewis structure for nh4+, the ammonium ion. Draw the lewis structure for ammonium, nh4+, include formal charges. The lewis structure of ammonium ion indicates that in nh4+ ion, the central nitrogen atom is covalently bonded with four h atoms with a total charge of. 456k views 10 years ago. 1.3k views 2 years ago lewis structure. It contains 3 polar covalent bonds and 1 coordinate covalent bond. Web draw lewis structures for covalent compounds. The following procedure can be used to construct lewis electron structures for more complex molecules and ions. Name of molecule ammonium ion; So nitrogen, on the periodic table, is in group 5, so it has 5 valence electrons. Web to draw lewis structures for molecules and polyatomic ions with one central atom. Web the ion is isoelectronic with methane and borohydride and has a tetrahedral structure. The diagram shows that the nh4+ ion has. Ammonium has a tetrahedral shape with bond angles of 109.5 o. Draw the lewis structure for the ammonium ion (nh4+). Web let's do the lewis structure for nh4+, the ammonium ion. Name of molecule ammonium ion; Draw the lewis structure for ammonium, nh4+, include formal charges. Web they follow the duet rule (2 electrons). It contains 3 polar covalent bonds and 1 coordinate covalent bond. Now that we have drawn the nh4+ lewis structure, let’s discuss what it means. The following procedure can be used to construct lewis electron structures for more complex molecules and ions. Web the nh4+ lewis structure is a representation of the ammonium ion, which consists of four hydrogen atoms bonded to a central nitrogen atom. This problem has been solved! Draw the lewis structure for the ammonium ion (nh4+). Web to draw lewis structures for molecules and polyatomic ions with one central atom. The following procedure can be used to construct lewis electron structures for more. I also go over hybridization, shape and bond angle. The following procedure can be used to construct lewis electron structures for more complex molecules and. The lewis structure of ammonium ion indicates that in nh4+ ion, the central nitrogen atom is covalently bonded with four h atoms with a total charge of. Web the ion is isoelectronic with methane and borohydride and has a tetrahedral structure.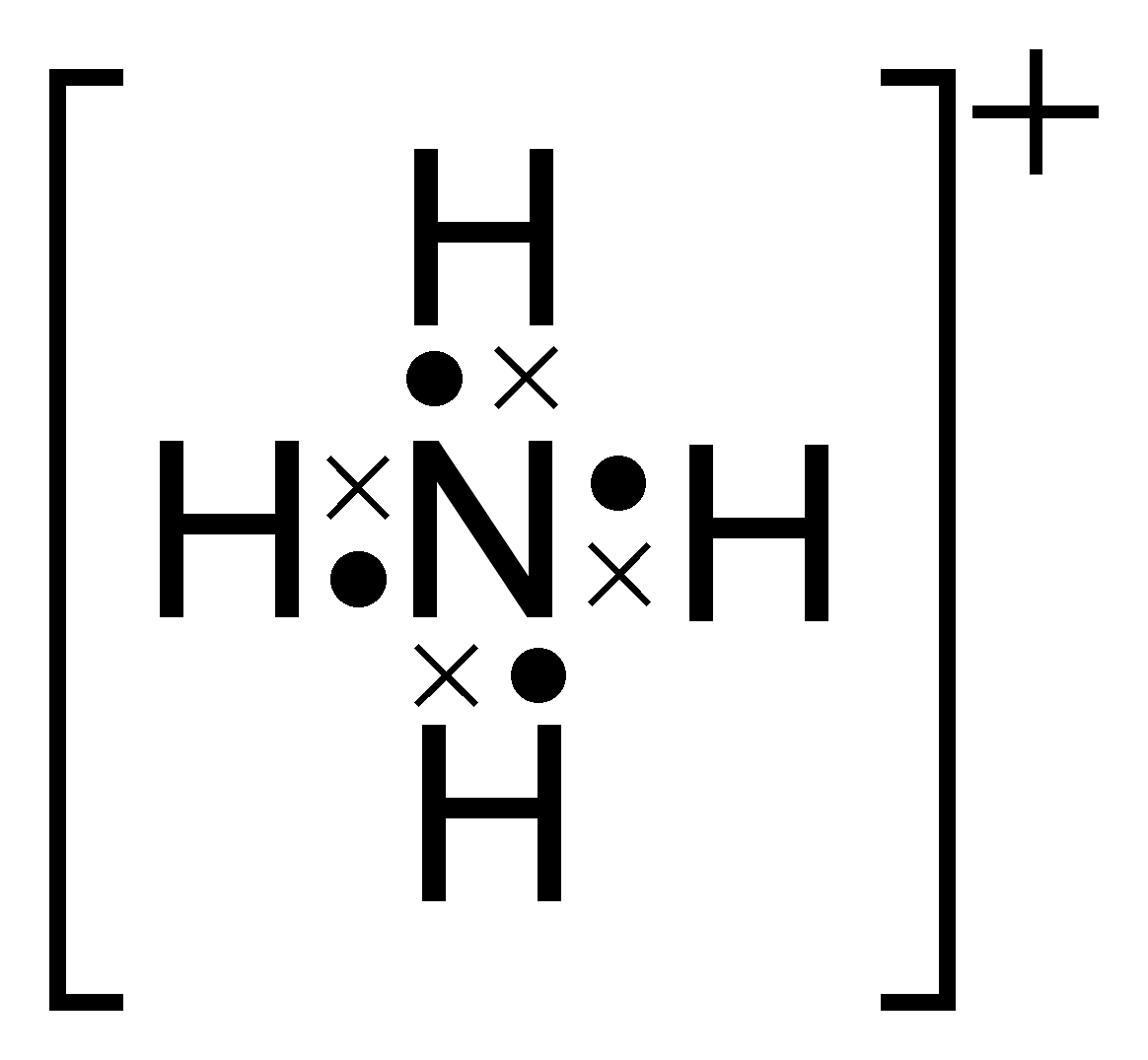
Electron Dot Diagram Of Ammonium Ion Wiring Diagram Pictures

NH4+ Lewis Structure (Ammonium Ion) in 2021 Lewis, Math equations, Nh 4
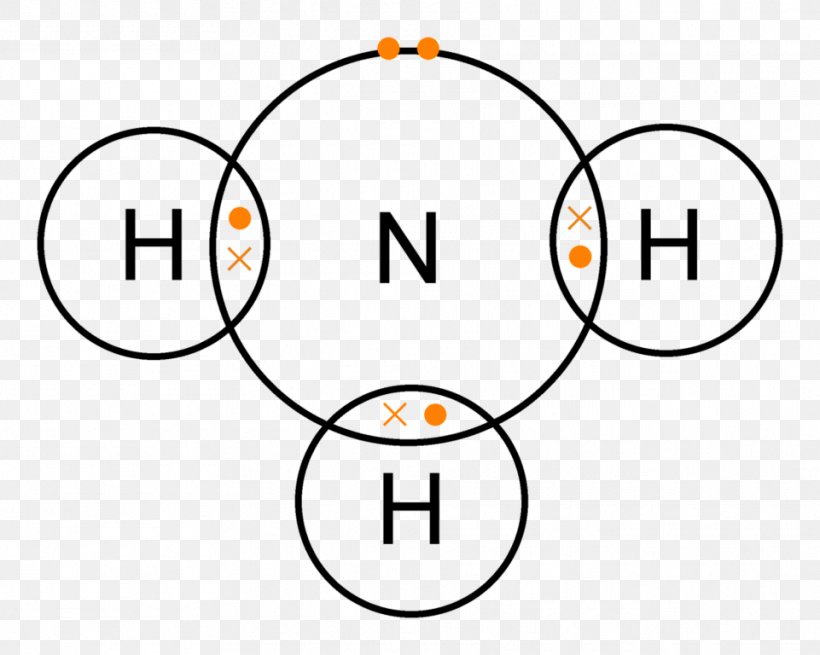
Lewis Structure Ammonia Covalent Bond Lone Pair Chemical Bond, PNG
This Video Explains Writing Lewis Structure Of.
So Nitrogen, On The Periodic Table, Is In Group 5, So It Has 5 Valence Electrons.
This Structure Is Commonly Used In.
A Lewis Structure Is A Way To Show How.
Related Post: