Draw The Lewis Structure For So2
Draw The Lewis Structure For So2 - How do you draw the lewis structure for ions? Calculate the total number of valence electrons. University professor with 10+ years. Do not consider ringed structures. We start with a valid lewis structure and then. Web sulfur dioxide molecule contains one sulfur atom and two oxygen atoms. Determine the total valence electrons. Explicitly showing the zero charges is optional. I have seen two different ways the lewis structure is written: The formal charges of the so 2 with the single bond and a double bond is larger than the so 2 with two double bonds. In order to draw the lewis structure of so2, first of all you have to find the total number of valence electrons present in the so2 molecule. Web by using the lewis concept we can draw the best resonate structure for sulfur dioxide. Web here are the steps i follow when drawing a lewis structure. Web sulfur dioxide molecule contains. The formal charges of the so 2 with the single bond and a double bond is larger than the so 2 with two double bonds. So i would assume that the one with two double bonds is the correct structure. Web sulfur dioxide molecule contains one sulfur atom and two oxygen atoms. Count the total number of valence electrons :. Here, a = central atom, x = surrounding atoms and e = the lone pairs. Sulfur dioxide | so 2. What are some examples of lewis structures? How to draw lewis structure for so2 ? This video solution was recommended by our tutors as helpful for the problem above. So2 is an ax2e type molecule, with 2 surrounding atoms i.e oxygen, and 1 lone pair of sulfur. This chemistry video tutorial explains how to draw the lewis structure of so2 also known as sulfur dioxide. 146k views 3 years ago. Decide which is the central atom in the structure. The lewis structure for so 2 requires you to place more than 8 valence electrons on sulfur (s). Web so2 lewis structure has a sulfur atom (s) at the center which is surrounded by two oxygen atoms (o). Calculate the total number of valence electrons. These valence electrons act as the building blocks of the structure. Do not consider ringed structures. We can understand the boding between atoms in a so2 molecule. Web what is the lewis structure for so2? Draw a lewis structure for so2 in which all atoms obey the octet rule. It discusses the molecular geometry, bond angle,. Web by using the lewis concept we can draw the best resonate structure for sulfur dioxide. So we are going to study how the best structure we can determined by knowing shape , hybridization etc. Draw a lewis structure for so2 in which all atoms have a formal charge of zero.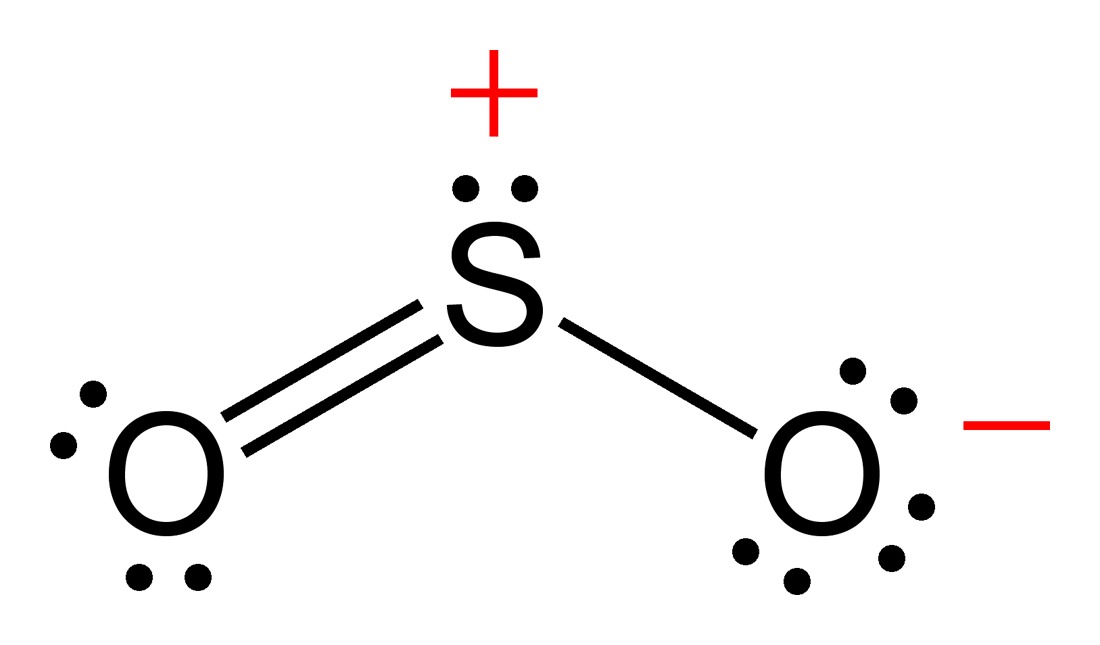
SO2(Sulfur Dioxide) Molecular Geometry & Lewis Structure Geometry of
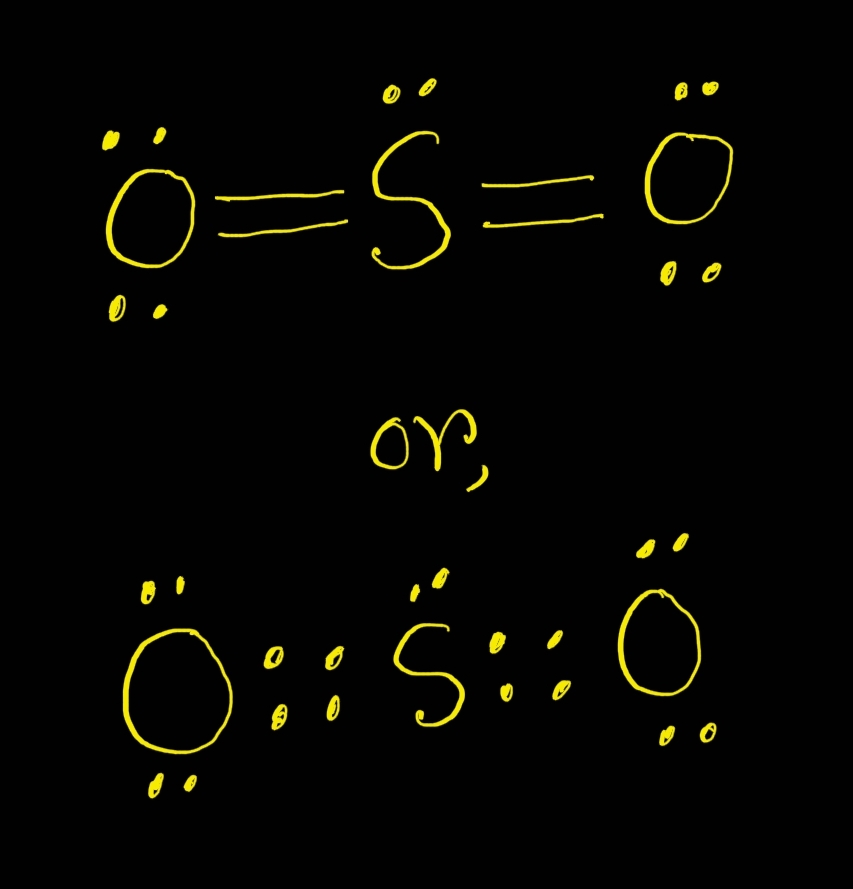
SO2 Lewis Structure ,Valence Electrons ,Formal Charge,Polar or Nonpolar
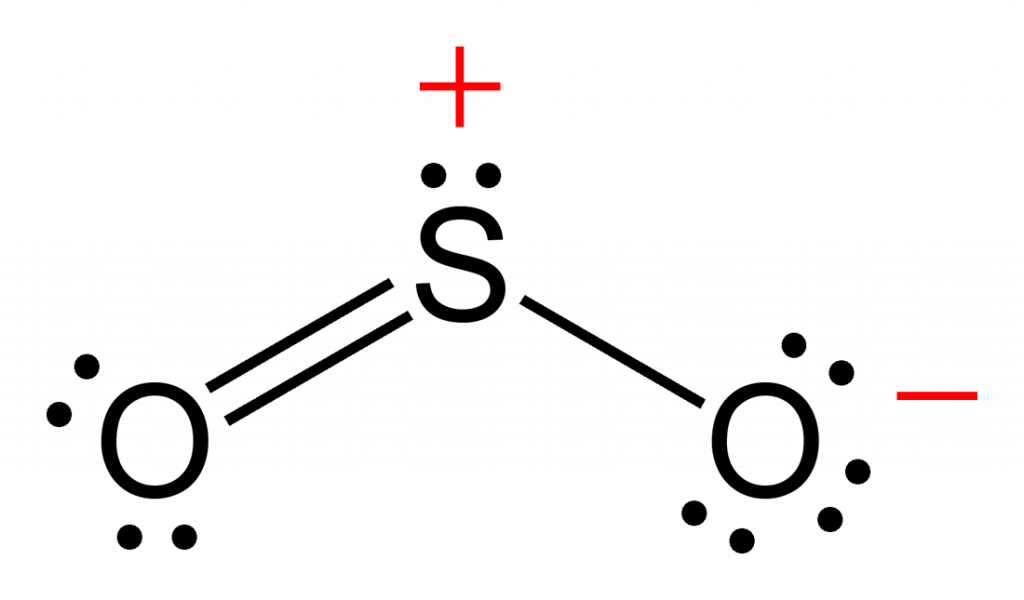
SO2(Sulfur Dioxide) Molecular Geometry & Lewis Structure Geometry of
Explicitly Showing The Zero Changes Is Optional.
Web To Draw The Lewis Structure Of The So A 2 (Sulfur Dioxide) Molecule, Follow These Steps:
Web Sulfur Dioxide Molecule Contains One Sulfur Atom And Two Oxygen Atoms.
How Do You Draw The Lewis Structure For Ions?
Related Post: