Draw The Lewis Structure For Sicl2Br2
Draw The Lewis Structure For Sicl2Br2 - This is done by adding the valence shell electrons of all the constituent atoms. Draw the molecule by placing atoms on the grid and connecting them with bonds. Web this video shows you how to draw the lewis dot structure for sicl2br2. Draw the molecule by placing atoms on the grid and connecting them with bonds. Web to draw lewis structures for molecules and polyatomic ions with one central atom. In this compound, one silicon atom is attached with two chlorine and two bromine atoms. Choose a suitable central atom for the compound. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Draw the molecule by placing atoms on the grid and connecting them with bonds. Draw the lewis structure for sicl, br2. Lewis structures show all of the valence electrons in an atom or molecule. Include all ch more button. Web lewis structures are visual representations of the bonds between atoms and illustrate the lone pairs of electrons in molecules. Lone pairs of electrons themselves are usually represented by dots around the atoms. To add the element si, either double click on. (please note, none of the solutions are using the expanded octet rule or formal charges) h 2. These diagrams are helpful because they allow us to predict the shape of the molecule and see the positions of each atom. Include all lone pairs of electrons. Web 226 views 1 year ago lewis structure. Write lewis structures for the following: Include all lone pairs of electrons. To add the element si, either double click on any atom and type the element symbol, or access a periodic table of elements from the more button. Web to draw the lewis dot structure for sicl2br2, let's first determine the total number of valence electrons. This is done by adding the valence shell electrons. Write lewis structures for the following: To add the element si, either double click on any atom and type the element symbol, or access a periodic table of elements from the more draw the molecule by placing atoms on the grid and connecting them with bonds. In this compound, one silicon atom is attached with two chlorine and two bromine atoms. This problem has been solved! The total number of valence electrons is calculated and used to form single bonds between si and each of the cl and br atoms. Draw the molecule by placing atoms on the grid and connecting them with bonds. Find the number of valence electrons for each atom. Include all ch more button. In order to find the total valence electrons in a sicl2br2 molecule, first of all you should know the valence electrons present in silicon atom, chlorine atom as well as bromine atom. Determine the total number of valence electrons in the molecule. Sicl2br2 is a chemical formula for dibromo dichloro silane. Web in this article, “sicl2br2 lewis structure”, lewis structure drawing, hybridization, shape, formal charge calculation with some detailed explanations are discussed briefly. To add the element si, either double click on any atom and type the element symbol, or access a periodic table of elements from the more more button button. 22k views 10 years ago. Become a study.com member to unlock this answer! Web chemistry questions and answers.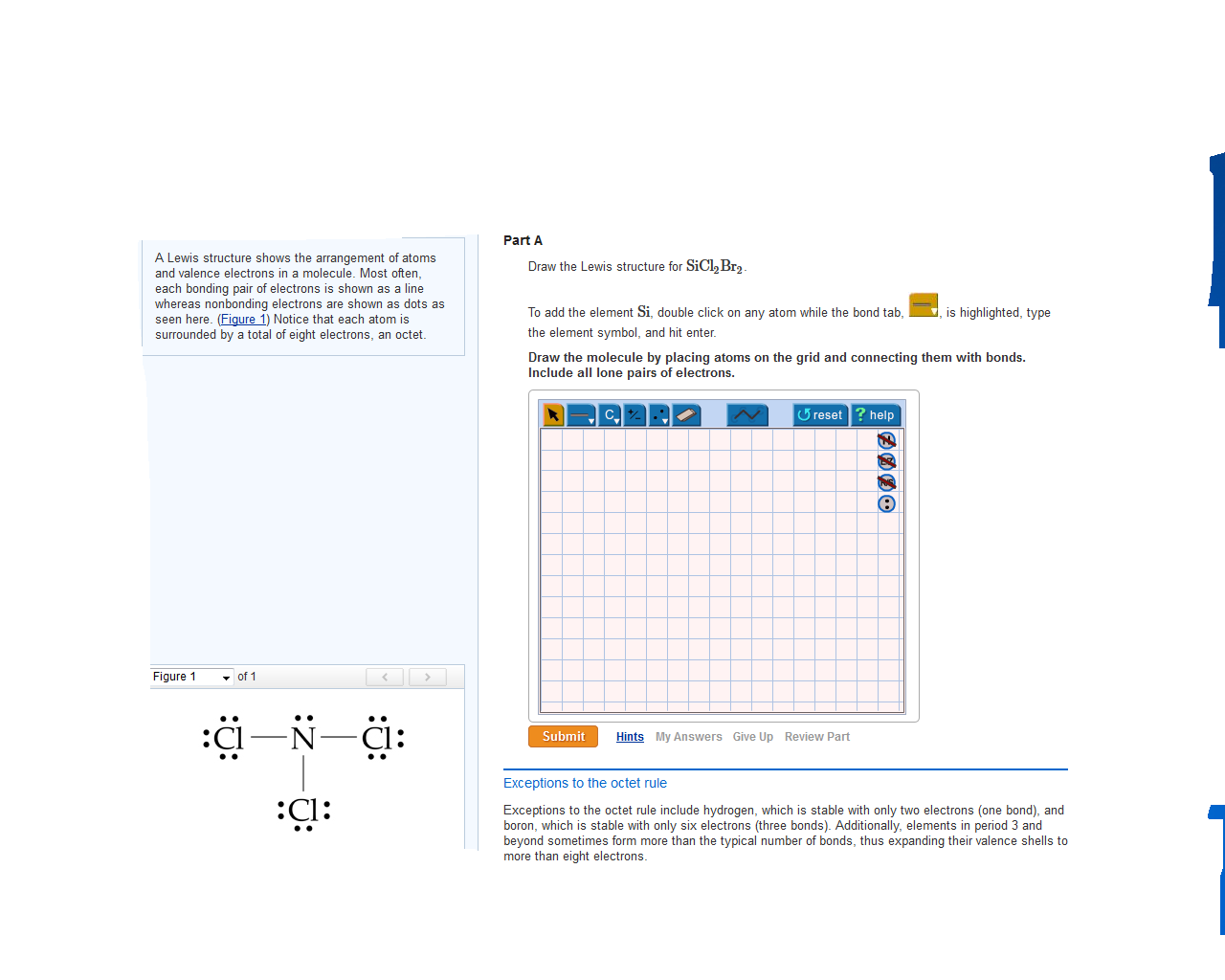
lewis structure for sicl2br2
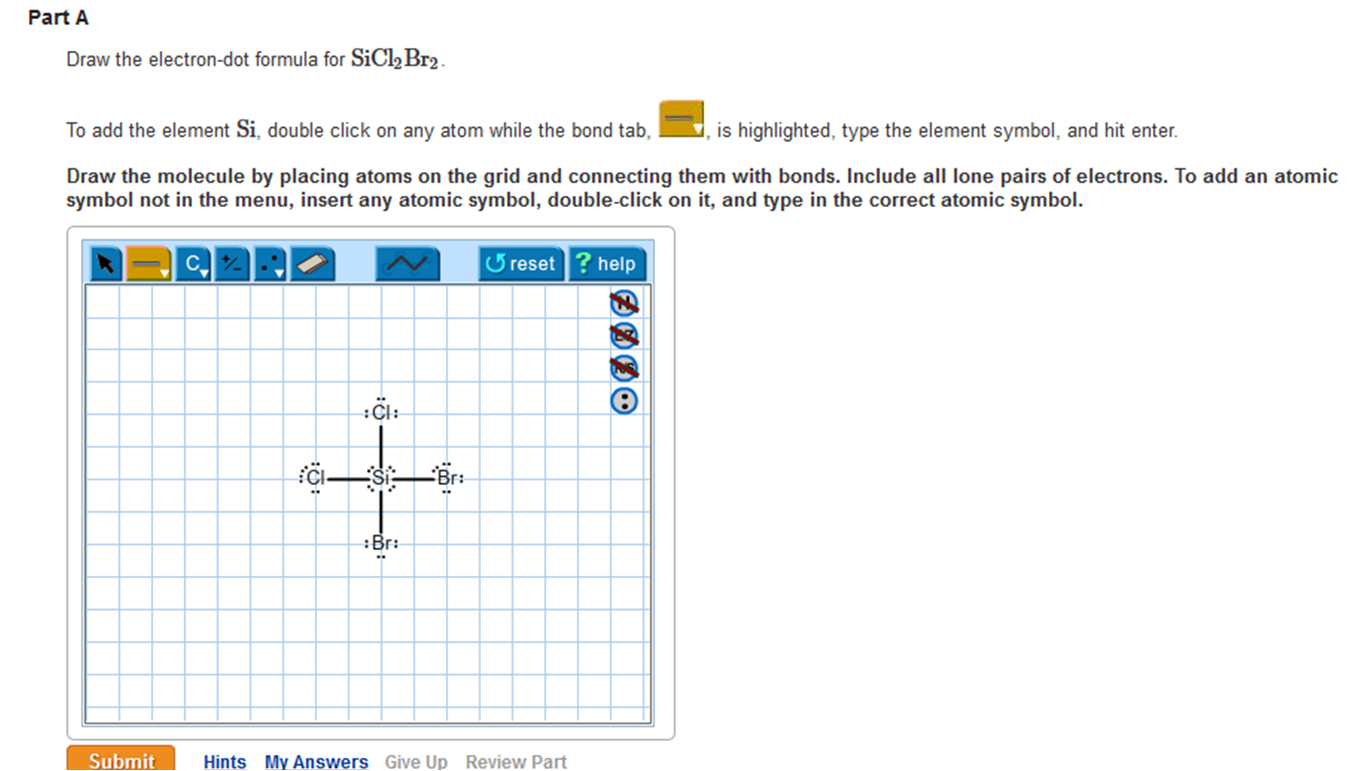
Solved Draw the electrondot formula for SiCl2Br2. This is
lewis structure for sicl2br2
So, If You Are Ready To Go With These 6 Simple Steps, Then Let’s Dive Right Into It!
This Is Done By Adding The Valence Shell Electrons Of All The Constituent Atoms.
Ed Lone Pairs Of Electrons.
Web Part A Draw The Lewis Structure For Sicl2Br2.
Related Post: