Draw The Lewis Structure For Nh4
Draw The Lewis Structure For Nh4 - 455k views 10 years ago. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Send feedback | visit wolfram|alpha. Web drawing the lewis structure for nh 4+. This article contains the nh4+ lewis structure and its hybridization, shape, bond angle, and detailed explanations. Web the nh4+ lewis structure is a representation of the ammonium ion, which consists of four hydrogen atoms bonded to a central nitrogen atom. #2 mention lone pairs on the atoms. (valence electrons are the electrons that are present in the outermost orbit of any atom.) The immune system then attacks the cells and destroys them, weakening the body. A lewis structure is a way to show how atoms share electrons when they form a molecule. For the central nitrogen atom: The number of lone pairs = the number of single bonds = the number of double bonds = 2. Br ch₂12 cio nh4 sih4 h₂o* give detailed solution with structures, don't…. A simple notation used to. Nitrogen, having 5 valence shell electrons, along with 4 from hydrogen, should have had 9 electrons. Because nh 4+ is a positive ion (called a cation) that means it lost one electron. Web here’s how you can easily draw the nh 4+ lewis structure step by step: Nh 4+ is one of the polyatomic ions that you should memorize. 455k views 10 years ago. Obeys the octet rule b. 66k views 3 years ago lewis structures. Web here, i have explained 6 simple steps to draw the lewis dot structure of nh4+ ion (along with images). Web draw the lewis structure for ammonium, nh4+, include formal charges. For the central nitrogen atom: Submit answer retry entire group 9 more group attempts remaining Web draw a lewis structure for nh4* in which the central n atom obeys the octet rule, and answer the following questions based on your drawing. Web identify the number of valence electrons in each atom in the \(\ce{nh4^{+}}\) ion. Web steps of drawing nh4+ lewis structure. Web the nh4+ lewis structure is a representation of the ammonium ion, which consists of four hydrogen atoms bonded to a central nitrogen atom. Based on this lewis structure, the calculated value for the formal charge on the nitrogen atom is a. Nitrogen (n) is in group 5 of the periodic table, so it has 5 valence electrons. Solution for 7) draw the correct lewis structure for the following molecular species: 66k views 3 years ago lewis structures. Find more chemistry widgets in wolfram|alpha. #3 if needed, mention formal charges on the atoms. The immune system then attacks the cells and destroys them, weakening the body. It's one of the few positive polyatomic ions you'll work with. Br ch₂12 cio nh4 sih4 h₂o* give detailed solution with structures, don't…. (valence electrons are the electrons that are present in the outermost orbit of any atom.) 455k views 10 years ago. Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle.
NH4+ Lewis Structure Lewis Dot Structure for NH4+ Ammonium ion

Draw the Lewis structure of `NH_(4)^(+)` ion . Sarthaks eConnect
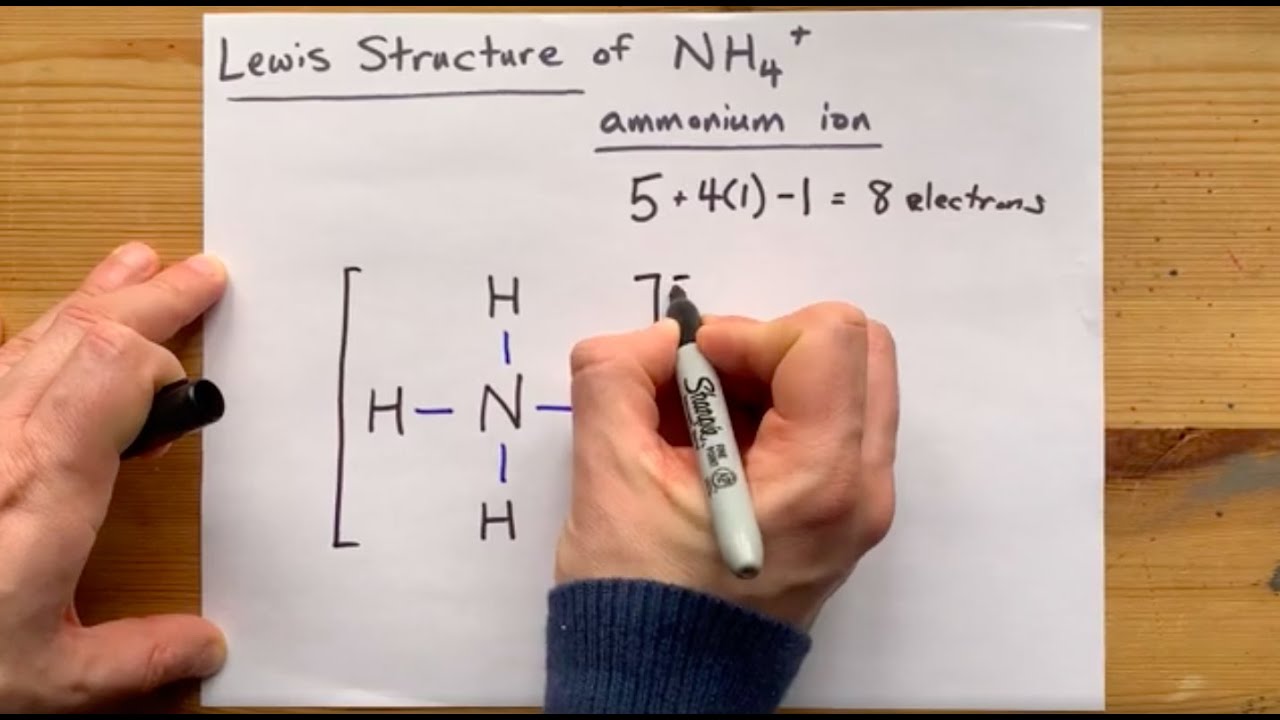
Lewis Structure of NH4+, Ammonium ion YouTube
So, If You Are Ready To Go With These 6 Simple Steps, Then Let’s Dive Right Into It!
In Nh4+ Lewis Structure , The Molecule Is Sp3 Hybridized And Has A Bond Angle Of 109.50, So The Molecule’s Shape Is.
Submit Answer Retry Entire Group 9 More Group Attempts Remaining
There Is A +1 Charge On Nitrogen Atom.
Related Post: