Draw The Lewis Structure For N2H4
Draw The Lewis Structure For N2H4 - For the n2h4 structure use the periodic table to find the total number of valence electrons for the. Calculate the total number of valence electrons. In order to draw the lewis structure of n2h2, first of all you have to find the total number of valence. Q h h 1 cho ns p|f br c | x more press (space to undo an action. Let’s break down each step in more detail. Include all lone pairs of electrons. Hydrogen (h) only needs two valence electrons to have a full outer shell. Draw a lewis structure for n2h4. Here, the given molecule is n2h2 (or dinitrogen dihydride). The valence electrons are the electrons in the. Thus far in this chapter, we have discussed the various types of bonds that form between atoms and/or ions. Hydrogen (h) only needs two valence electrons to have a full outer shell. Web draw lewis structures depicting the bonding in simple molecules. 2.1k views 1 year ago lewis structure. Note the relative energies of the orbitals. In the n 2 h 4 lewis structure the two nitrogen (n) atoms go in the center (hydrogen always goes on the outside). Here, the given molecule is n2h2 (or dinitrogen dihydride). For the n2h4 structure use the periodic table to find the total number of valence electrons for the. Include all lone pairs of electrons and hydrogen atoms. N. N 2 h 4 is straightforward with no double or triple bonds. Web drawing the lewis structure for n2h4. N 2 h 2 is straightforward with no double or triple bonds. The molecular geometry for the n2h4 molecule is trigonal pyramidal and the electron geometry is tetrahedral. Draw the molecules by placing atoms on the grid and connecting them with. What is the hybridization of n in hydrazine? Web n2h4 lewis structure is made up of two nitrogen (n) and four hydrogens (h) having two lone pairs on the nitrogen atoms (one lone pair on each nitrogen) and containing a total of 10 shared electrons. Draw the lewis structures of n_2h_4, n_2h_2, and n_2. Lewis dot structures are schematic representations of valence electrons and bonds in a molecule. Note the relative energies of the orbitals. In the n 2 h 2 lewis structure the two nitrogen (n) atoms go in the center (hydrogen always goes on the outside). Include all lone pairs of electrons. Calculate the total number of valence electrons. Draw a lewis structure for n2h4. C) draw the orbital diagram for the valence electrons of nitrogen in a n2h4 molecule below. #1 draw a rough sketch of the structure. How to draw a lewis structure for n2h4? There are 4 steps to solve this one. A) draw the lewis structure for hydrazine, n2h4 b) list the electronic and molecular geometries for nh3. Here’s the best way to solve it. Hydrogen (h) only needs two valence electrons to have a full outer shell.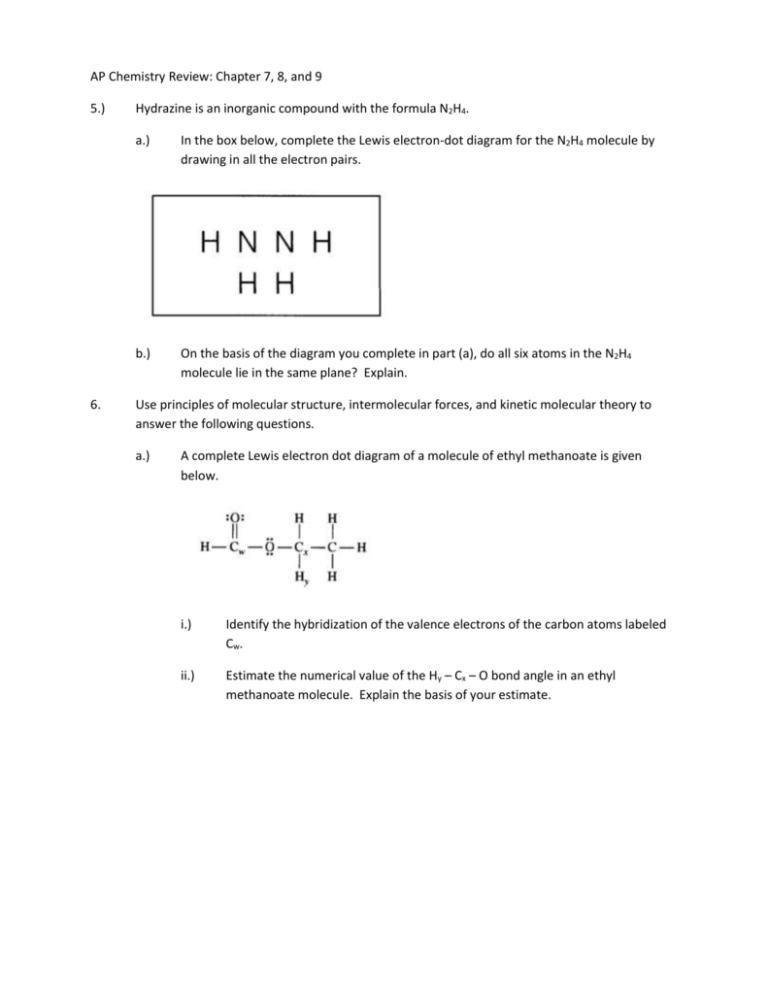
The Lewis Structure Of N2h4 Draw Easy
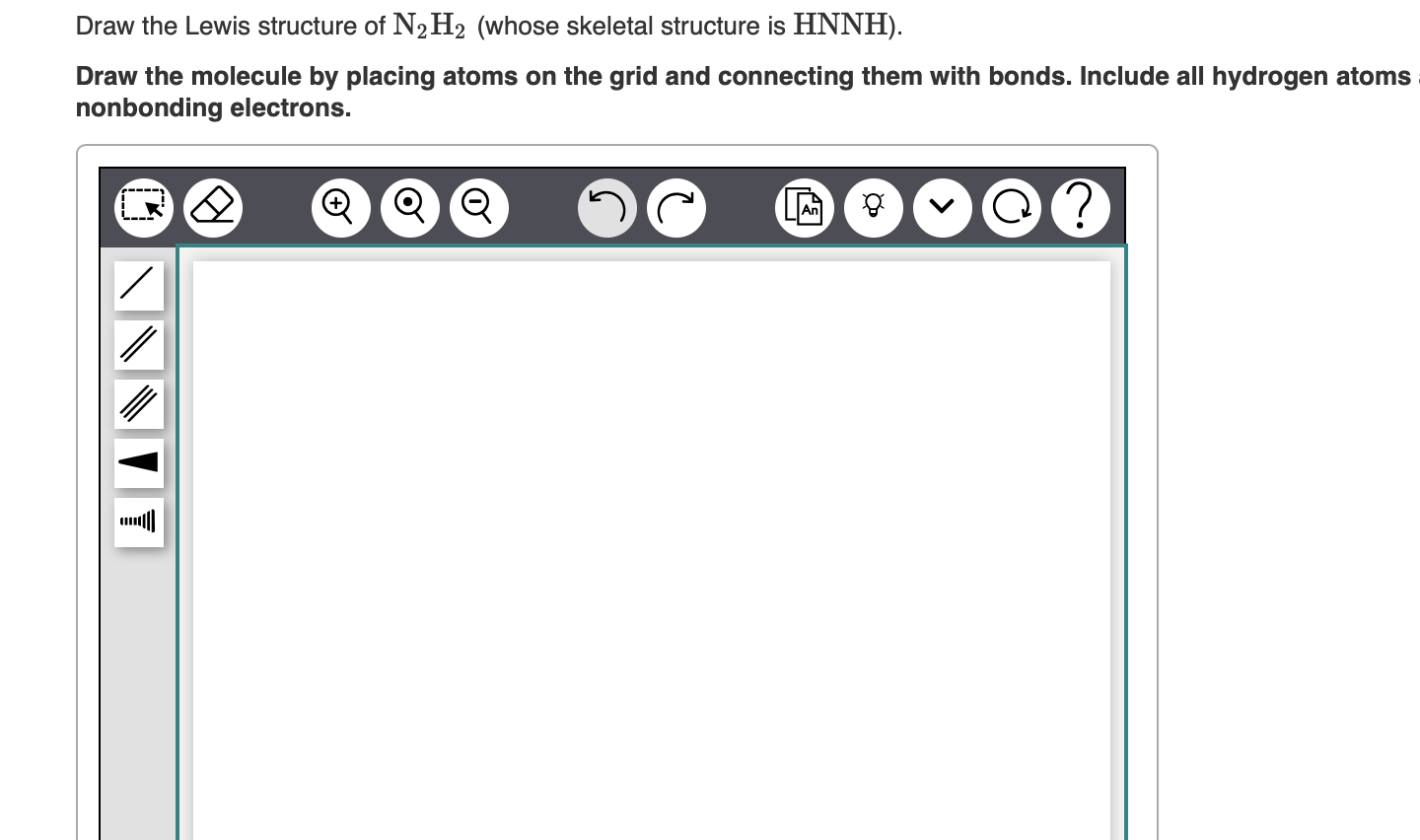
Solved Draw the Lewis structure of N2H4 (whose skeletal
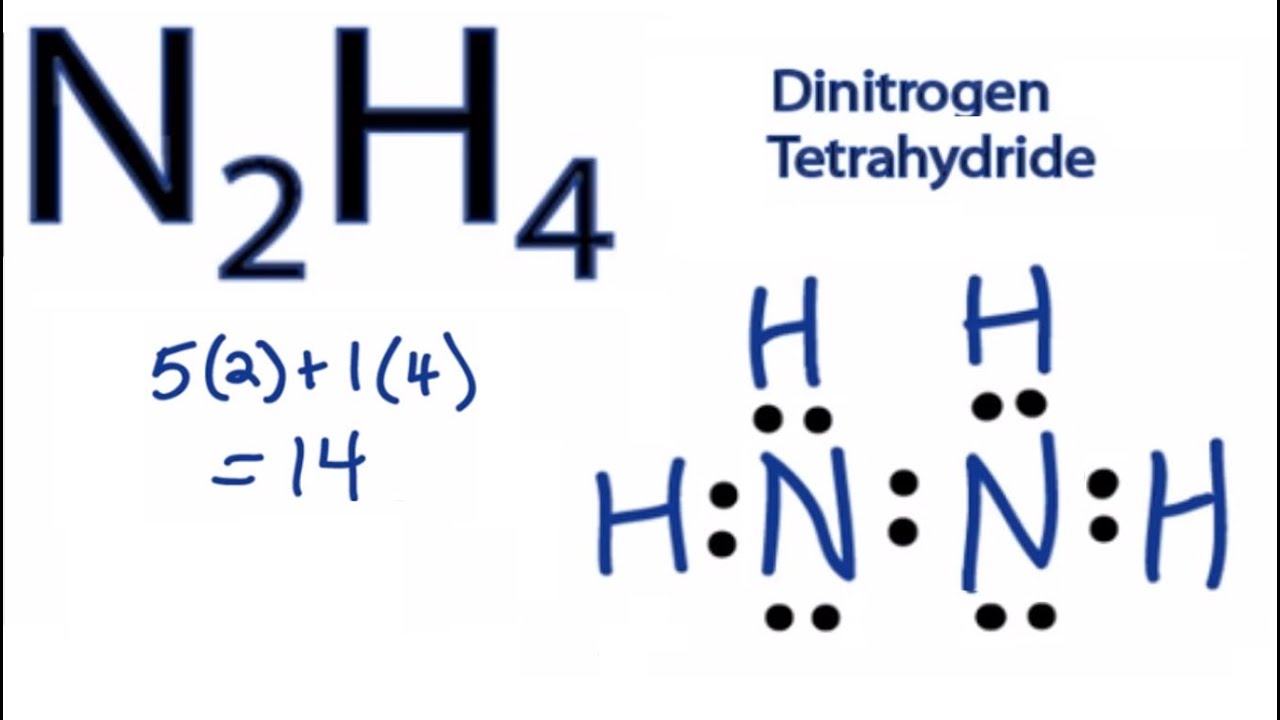
N2H4 Lewis Structure How to Draw the Lewis Structure for N2H4 YouTube
Web Chemistry Questions And Answers.
Web Drawing The Lewis Structure For N.
Lewis Structures Show All Of The Valence Electrons In An Atom Or Molecule.
Thus Far In This Chapter, We Have Discussed The Various Types Of Bonds That Form Between Atoms And/Or Ions.
Related Post: