Draw The Lewis Structure For A Peroxide Ion
Draw The Lewis Structure For A Peroxide Ion - Determine the net number of and. Both oxygen atoms are joint through a single bond. No one rated this answer yet — why not be the first? This problem has been solved! Web to use the lewis structure calculator follow these steps: Write the electron configuration for o2, o2 (superoxide ion) and o22 (peroxide ion). Atom formal charge left o right o. Enter the formula of the molecule in the field provided for it. Li2o2 130 pm to bao2 147 pm. Furthermore, the peroxide ion is diamagnetic. No one rated this answer yet — why not be the first? Student proposes the following lewis structure for the peroxide (o22) ion. The peroxide ion, o₂²⁻, consists of two oxygen atoms bonded together with a single covalent bond, where each oxygen atom has one unpaired electron. Draw the mo diagram for molecular oxygen, o2. Enter the formula of the. Web these two electrons, according to the molecular orbital theory, complete the two π* antibonding orbitals. This problem has been solved! Li2o2 130 pm to bao2 147 pm. And a lewis structure for −o− o−, which clearly derives from hydrogen peroxide, h −o −o −h, which is. This problem has been solved! Lewis dot structure of polyatomic ions. This problem has been solved! When drawing the structure of an ion, be sure to add/subtract electrons to account for. Draw lewis structures depicting the bonding in simple molecules. Draw the lewis structure for a peroxide (0,) ion. Student proposes the following lewis structure for the peroxide (o22) ion. This problem has been solved! Web these two electrons, according to the molecular orbital theory, complete the two π* antibonding orbitals. Furthermore, the peroxide ion is diamagnetic. Li2o2 130 pm to bao2 147 pm. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. When drawing the structure of an ion, be sure to add/subtract electrons to account for. This problem has been solved! Write lewis symbols for neutral atoms and ions. Atom formal charge left o right o. Thus far in this chapter, we have discussed the various types of bonds that form between atoms and/or ions. There were 2 ×1(h) +2 ×6(o) valence electrons, i.e. Draw the lewis structure for a peroxide (0,) ion. By the end of this section, you will be able to: 10k views 3 years ago lewis structures. For the peroxide ion, oxygen has six valence electrons.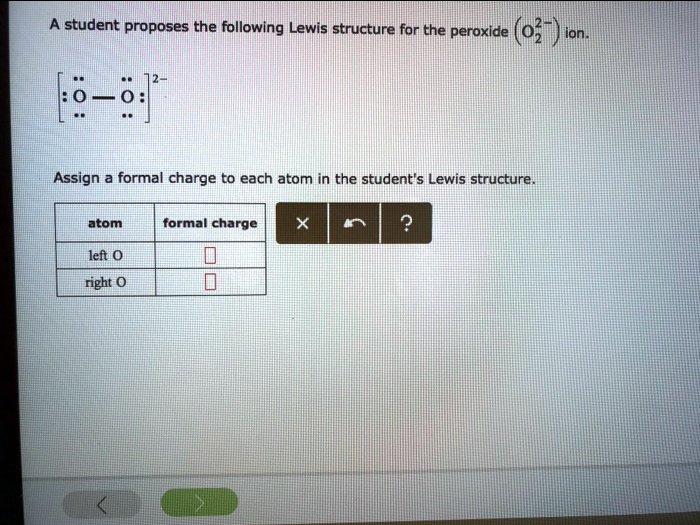
SOLVED A student proposes the following Lewis structure for the
How To Draw A Lewis Structure vrogue.co

How to Draw a Lewis Structure
For The Peroxide Ion, Oxygen Has Six Valence Electrons.
7 Electron Pairs To Distribute Over 4 Centres.
Web A Video Explanation Of How To Draw The Lewis Dot Structure For The Peroxide Ion, Along With Information About The Compound Including Formal Charges, Polarity.
Determine The Net Number Of And.
Related Post: