Draw The Lewis Dot Structure For H2O
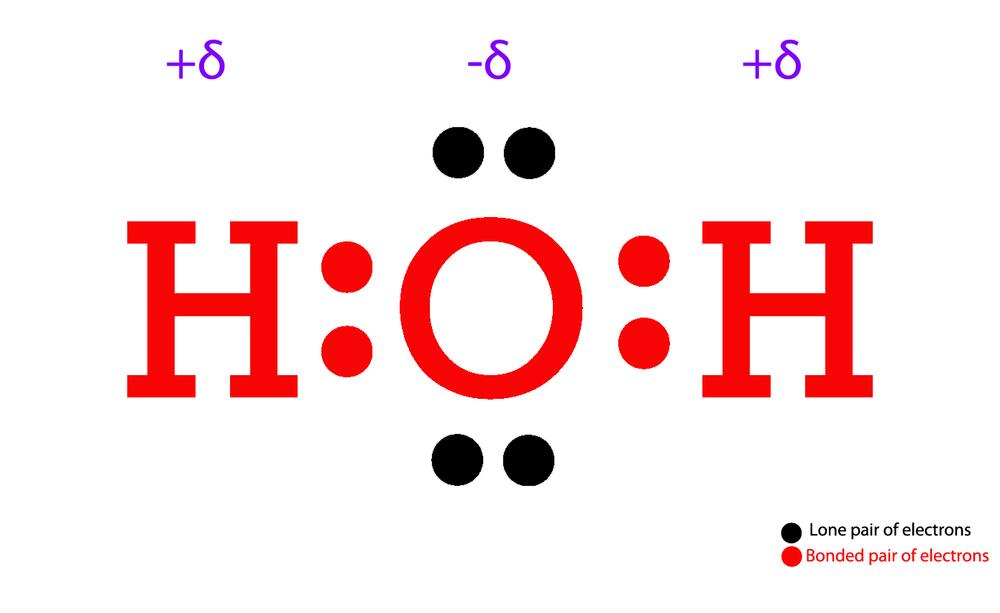
Draw The Lewis Dot Structure For H2O - For h₂o, o must be the central atom. It is eight to form a single h2o molecule. What are some examples of lewis structures? I also go over hybridization,. Determine the total number of valence electrons in the molecule or ion. How to draw lewis structure for h 2 o; Web we can illustrate the formation of a water molecule from two hydrogen atoms and an oxygen atom using lewis dot symbols: You can find a procedure for drawing lewis structures at this location. The example is for the nitrate ion. Try structures similar to h 2 o (like hcl or ch 4) for more practice. How do you draw the lewis structure for ions? Lewis structure of h2o (or water) contains single bonds between the oxygen (o) atom and each hydrogen (h) atom. What are some examples of lewis structures? This is the lewis dot structure for h2o. For the h2o structure use the periodic table to find the total number of valence electrons for. The following procedure can be used to construct lewis electron structures for more complex molecules and ions. 162k views 12 years ago every video. You could alternatively also draw the structure by including two dots for every bond. How do you draw the lewis structure for ions? For the h2o structure use the periodic table to find the total number. Web make sure you put the correct atom at the center of the water (h 2 o) molecule. The video covers the basic lewis structures you'll. Representing valence electrons with dots. You must arrange 8 electrons in pairs so that o has 8 and each h has two electrons in its valence shell. It is eight to form a single. You can find a procedure for drawing lewis structures at this location. Thus far in this chapter, we have discussed the various types of bonds that form between atoms and/or ions. Molecular geometry of h 2 o;. How to draw lewis structure for h 2 o; For the h2o structure use the periodic table to find the total number of valence electrons for the h2o. Web lewis dot structures also called electron dot structures are diagrams that describe the chemical bonding between atoms in a molecule. Web we can illustrate the formation of a water molecule from two hydrogen atoms and an oxygen atom using lewis dot symbols: What is an example of a lewis structures practice problem? It is eight to form a single h2o molecule. They also display the total number of lone pairs present in each of the atoms that constitute the molecule. The following procedure can be used to construct lewis electron structures for more complex molecules and ions. For the h2o structure use the periodic table to find the total number of. Determine the total number of valence electrons in the molecule or ion. Web what are lewis dot structures used for? You could alternatively also draw the structure by including two dots for every bond. Write lewis symbols for neutral atoms and ions.
H2O Lewis Structure, Molecular Geometry, and Hybridization
Draw The Lewis Structure Of H2o

H2O Lewis structure and Molecular Geometry [No1 Best Explanation
Web Lewis Structure Of Water Molecule Contains Two Single Bonds Around Oxygen Atom.
Using Lewis Dot Symbols To Describe Covalent Bonding.
Draw Lewis Structures Depicting The Bonding In Simple Molecules.
How Do You Draw The Lewis Structure For Ions?
Related Post: