Draw The Electrondot Formula For The Element Sulfur
Draw The Electrondot Formula For The Element Sulfur - Web a lewis electron dot diagram (or electron dot diagram, or a lewis diagram, or a lewis structure) is a representation of the valence electrons of an atom that uses. Web the dot structure for sulfur dioxide has sulfur with a double bond to an oxygen on the left, and two lone pairs of electrons on that oxygen, and the sulfur with a. Include all of the valence electrons. You can add the valence electrons by clicking on the. You can add the valence electrons by clicking on the button and selecting the atom. We have 6 valence electrons for sulfur, but we have 2 sulfurs; Send feedback | visit wolfram|alpha. And we have 7 valence electrons for chlorine, but there are 2. You'll get a detailed solution from a subject matter expert that helps you. Since fluorine is found in group 7a of the periodic table, it contains 7 valence electrons. In the case of sulfur, which is an element in the periodic table with atomic number 16, it has six valence. And we have 7 valence electrons for chlorine, but there are 2. Send feedback | visit wolfram|alpha. 639k views 9 years ago lewis structures. Create an account to view solutions. We have 6 valence electrons for sulfur, but we have 2 sulfurs; 82k views 10 years ago. You can add the valence electrons by clicking on the or button and clicking the atom. This problem has been solved! Create an account to view solutions. Web as usual, we will draw two dots together on one side, to represent the 2s electrons. This problem has been solved! Web the dot structure for sulfur dioxide has sulfur with a double bond to an oxygen on the left, and two lone pairs of electrons on that oxygen, and the sulfur with a. Create an account to view. You can add the valence electrons by clicking on the. This problem has been solved! Include all of the valence electrons. Added jun 9, 2014 by webtester in chemistry. Web a lewis electron dot symbol (or electron dot diagram or a lewis diagram or a lewis structure) is a representation of the valence electrons of an atom that uses dots around. You can add the valence electrons by clicking on the or button and. In the case of sulfur, which is an element in the periodic table with atomic number 16, it has six valence. Since fluorine is found in group 7a of the periodic table, it contains 7 valence electrons. Web as usual, we will draw two dots together on one side, to represent the 2s electrons. This is the s2cl2 lewis structure. And we have 7 valence electrons for chlorine, but there are 2. This widget gets the lewis structure of chemical compounds. You'll get a detailed solution from a subject matter expert that helps you. However, conventionally, we draw the dots for the two p electrons on different sides. We have 6 valence electrons for sulfur, but we have 2 sulfurs; Create an account to view solutions.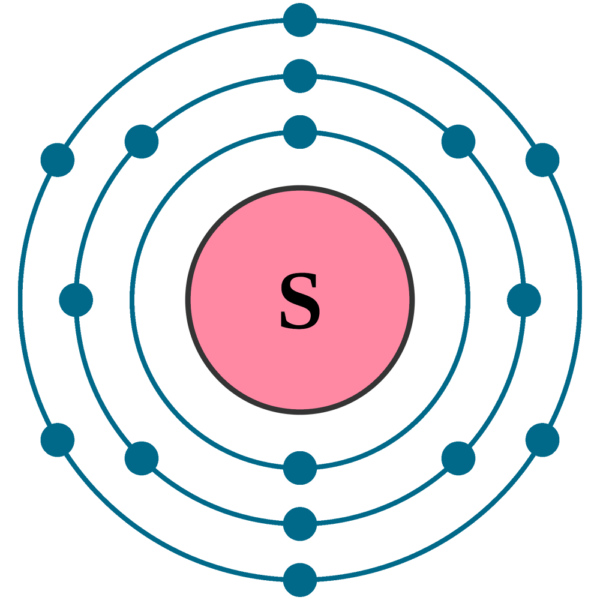
Sulfur S (Element 16) of Periodic Table Elements FlashCards

Sulfur Definition, Facts, Symbol, Allotropes, Properties, Uses
:max_bytes(150000):strip_icc()/sulfuratom-58b602563df78cdcd83d5a9d.jpg)
Atom Diagrams Electron Configurations of the Elements
Web Draw A Valid Electron Dot Structure For Each Of The Given Elements.
639K Views 9 Years Ago Lewis Structures.
Web The Dot Structure For Sulfur Dioxide Has Sulfur With A Double Bond To An Oxygen On The Left, And Two Lone Pairs Of Electrons On That Oxygen, And The Sulfur With A.
You Can Refer To The Periodic Table If Necessary.
Related Post: