Draw The Electron Dot Formula For The Element Sulfur
Draw The Electron Dot Formula For The Element Sulfur - This problem has been solved! Lewis electron dot diagrams for ions have fewer (for cations) or more (for anions) dots than the corresponding atom. The number of dots equals the number of valence electrons in the atom. C= single bond on the left. 2 a simplified way to show valence electrons. Web remember each bond is 2 electrons, and each lone pair is 2 electrons. It has 2 of these nonbonding, and a total of 8 bonding valence electrons which we'll divide by 2. There are four covalent bonds in the skeleton structure for sf 4. / [] this problem has been solved! The number of dots equals the number of valence electrons in the atom. You can add the valence electrons by clicking on the or button and clicking the atom. 3 x 6 = 18 valence electrons. This problem has been solved! 82k views 10 years ago. Lewis electron dot diagrams use dots to represent valence electrons around an atomic symbol. Because this requires using eight valence electrons to form the covalent bonds that hold the molecule together, there are 26 nonbonding valence electrons. C= single bond on the left. Web draw a valid electron dot structure for each of the given elements. The number of dots equals the number of valence electrons in the atom. Web calculate the formal charge. Web sulfur has 6 valence electrons if you look at the periodic table. The periodic table shows you how many valence electron each element has. Because this requires using eight valence electrons to form the covalent bonds that hold the molecule together, there are 26 nonbonding valence electrons. Shared pairs of electrons are drawn as lines between atoms, while lone. Added jun 9, 2014 by webtester in chemistry. Lewis electron dot diagrams for ions have fewer (for cations) or more (for anions) dots than the corresponding atom. The periodic table shows you how many valence electron each element has. Web a lewis electron dot diagram (or electron dot diagram or a lewis diagram or a lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. A lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. Remember that formal charge is calculated by taking the # of valence electrons, minus the lone electrons and the bonds, and we show that charge next to the molecule. 8 + (6 × × 7) = 50; Include all of the valence electrons. Know the importance of lewis dot in bonding. Web we draw the dot structure in the exact same manner, and then calculate the formal charges for the atoms in the molecule. There are four covalent bonds in the skeleton structure for sf 4. Lewis electron dot diagrams use dots to represent valence electrons around an atomic symbol. 3 all the elements in a column have the same electron dot diagram. In this case, we can condense the last few steps, since not all of them apply. The number of dots equals the number of valence electrons in the atom. 6 + 4(7) = 34.
Sulfur Bohr Model — Diagram, Steps to Draw Techiescientist
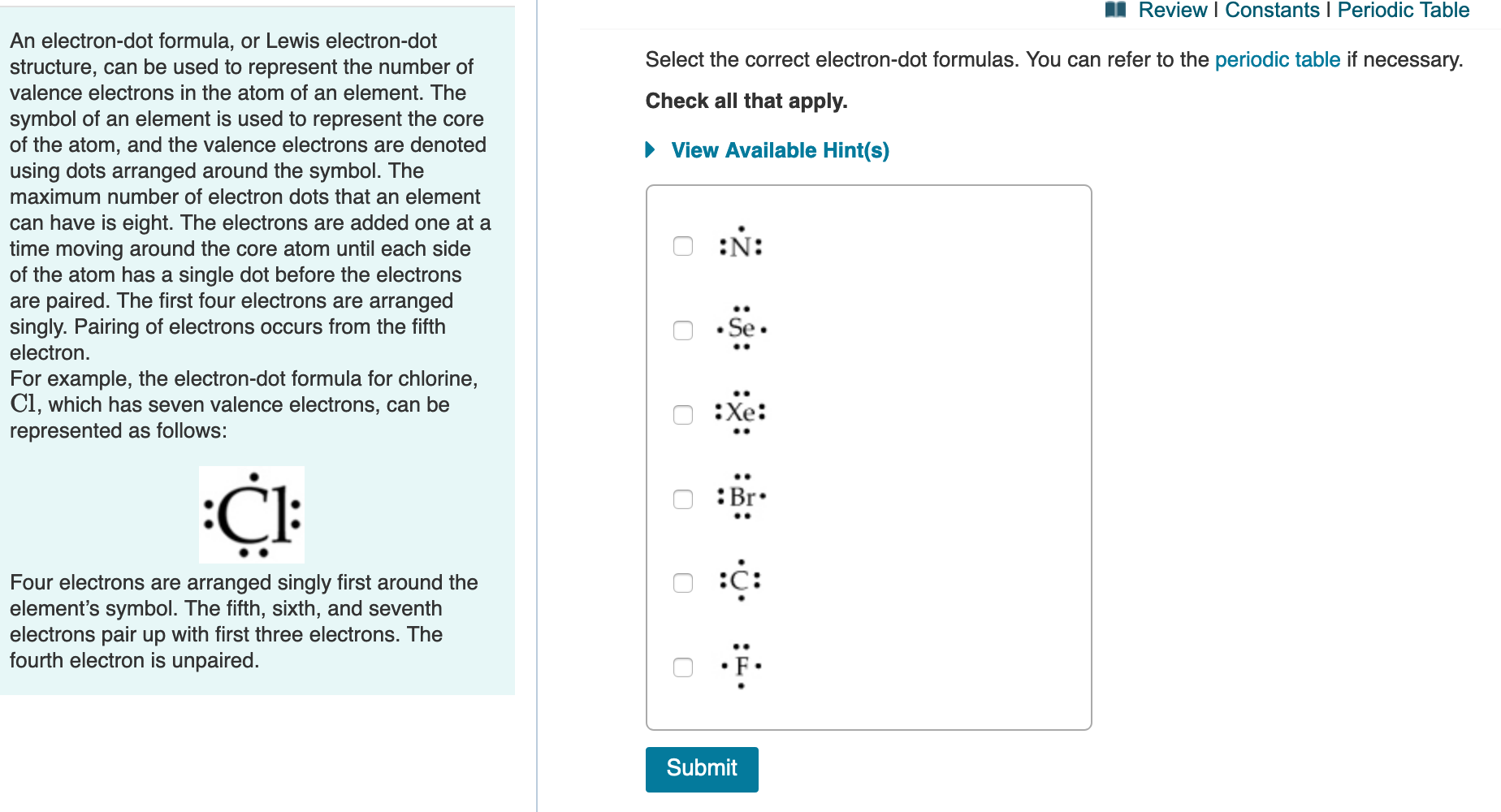
Solved Draw the electrondot formula for the element
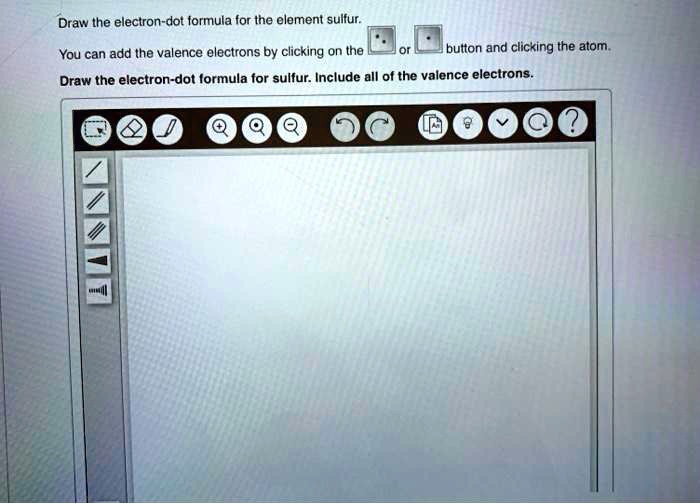
SOLVED Draw the electrondot formula for the element sulfur. You can
View Available Hint (S) ?
You Can Add The Valence Electrons By Clicking On The Or Button And Clicking The Atom.
Web The Valence Electrons Are The Electrons In The Outermost Shell.
For Representative Elements, The Number Of Valence Electrons Equals The Group Number On The Periodic Table.
Related Post: