Draw The Electron Configuration For A Neutral Atom Of Vanadium
Draw The Electron Configuration For A Neutral Atom Of Vanadium - [ar]4s 2 3d 3, 5 valence electrons. By knowing the electron configuration of an element, we can predict and. By the end of this section, you will be able to: Identify and explain exceptions to. [ar]4s 1 3d 5, 6 valence electrons. Asked feb 24, 2016 at 18:28. Web the full electron configuration of this element is 1s2 2s2 2p6 3s2 3p6 3d3 4s2 and its short, abbreviated, or simplified electron configuration is [ar]3d34s2. Web the electron configuration of sodium is 1s22s22p63s1 1 s 2 2 s 2 2 p 6 3 s 1 (table 2.7.1 2.7. [ar]4s 2 3d 2, 4 valence electrons. [ar]4s 2 3d 5, 7 valence electrons. Titanium ← vanadium → chromium. By the end of this section, you will be able to: Electrons always fill in the lowest energy. Web the electron configuration of sodium is 1s22s22p63s1 1 s 2 2 s 2 2 p 6 3 s 1 (table 2.7.1 2.7. 1s2 2s2 2p6 3s2 3p6 3d3 4s2. By the end of this section, you will be able to: [ar]4s 2 3d 2, 4 valence electrons. [ar]4s 2 3d 3, 5 valence electrons. This problem has been solved! Electrons always fill in the lowest energy. Web here's the electron configuration for vanadium: Electrons always fill in the lowest energy. [ar]4s 2 3d 3, 5 valence electrons. This problem has been solved! Identify and explain exceptions to. [ar]4s 2 3d 3, 5 valence electrons. Electrons always fill in the lowest energy. The electron configuration describes the arrangement of electrons in an atom’s energy. Web the electronic configuration of vanadium can be represented as: 1s² (2 electrons) 2s² (2 electrons) 2p⁶ (6 electrons) 3s² (2 electrons) 3p⁶ (6 electrons) 4s² (2 electrons) 3d³ (3 electrons) so,. Web the full electron configuration of this element is 1s2 2s2 2p6 3s2 3p6 3d3 4s2 and its short, abbreviated, or simplified electron configuration is [ar]3d34s2. Titanium ← vanadium → chromium. 1s2 2s2 2p6 3s2 3p6 4s2 3d3. [ar]4s 2 3d 5, 7 valence electrons. In this section we shall assign electrons to orbitals and come up with some rules to determine the ground state electron configuration of atoms of the different. By knowing the electron configuration of an element, we can predict and. Vanadium has an electron configuration of [ar] 3d^3 4s^2. Draw the electron configuration for a neutral atom of sodium. Web here's the electron configuration for vanadium: Vanadium has an atomic number of 23, which means it has 23. [ar]4s 1 3d 5, 6 valence electrons.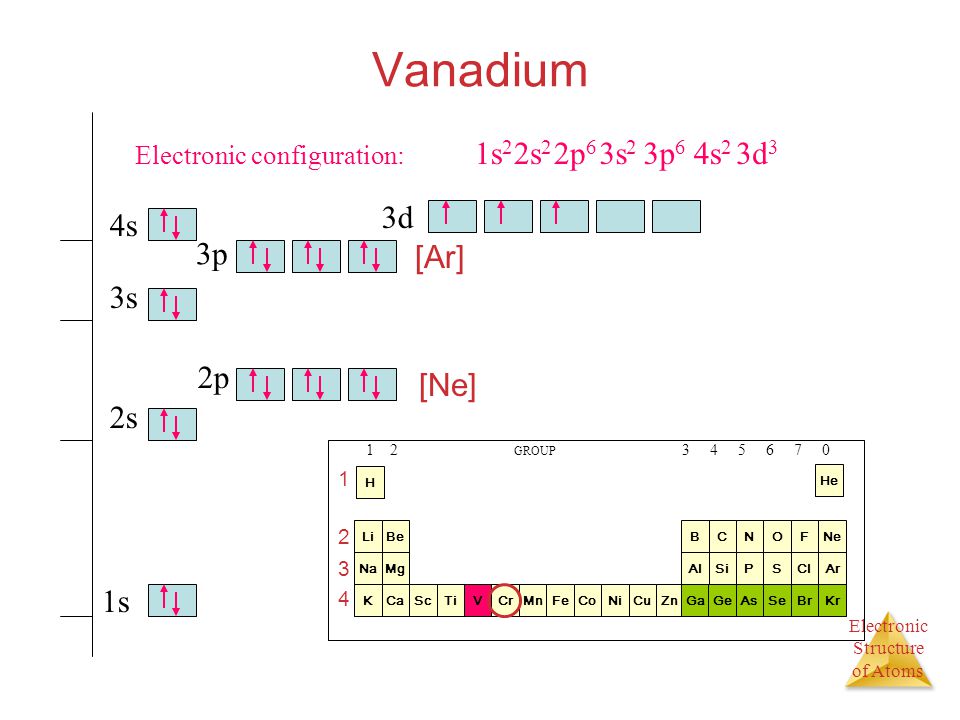
Vanadium Electron Configuration (V) with Orbital Diagram

Vanadium Facts
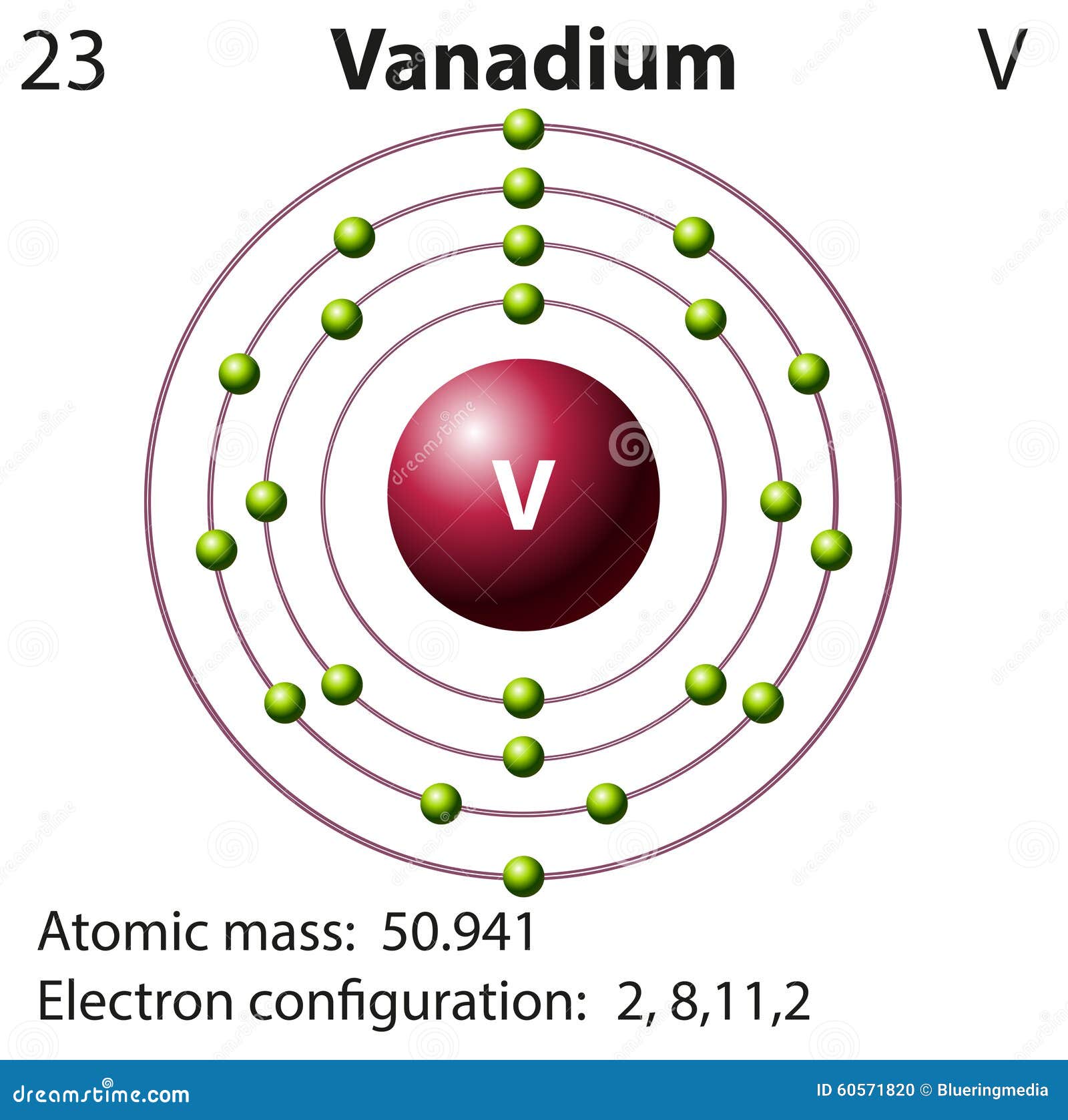
Symbol and Electron Diagram for Vanadium Stock Vector Illustration of
Web 68K 12 201 386.
The Electron Configuration For A Neutral Vanadium Atom Is 1S²2S²2P⁶3S²3P⁶4S²3D³.
This Problem Has Been Solved!
Web The Electron Configuration Of Sodium Is 1S22S22P63S1 1 S 2 2 S 2 2 P 6 3 S 1 (Table 2.7.1 2.7.
Related Post: