Draw The Electron Configuration For A Neutral Atom Of Oxygen
Draw The Electron Configuration For A Neutral Atom Of Oxygen - Web electron configurations are an organized means of documenting the placement of electrons based upon the energy levels and orbitals groupings of the periodic table. Remember, a neutral atom contains the same number of protons and electrons. 1s 2 2s 2 2p 4 (for an atom). Electron configurations list every subshell for an atom or ion and how many electrons are in each subshell. Web oxygen (atomic number 8) has a pair of electrons in any one of the 2 p orbitals (the electrons have opposite spins) and a single electron in each of the other two. Web the upper right side shows the number of electrons in a neutral atom. Web the arrangement of electrons in oxygen in specific rules in different orbits and orbitals is called the electron configuration of oxygen. Look at the periodic table and find an atomic number of oxygen, which is 8. In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. Fill these 8 electrons in the following order:. Web this means that the electron configuration of a neutral oxygen atom must account for 8 electrons. Draw a lewis electron dot diagram for an atom or a monatomic ion. Web consider what results when a proton, h+, becomes bonded to methanol by way of one of the unshared electron pairs on the oxygen atom, i.e., this time we use. In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. Previously we discussed the concept of electron shells, subshells,. Web the arrangement of electrons in oxygen in specific rules in different orbits and orbitals is called the electron configuration of oxygen. Web this means that the electron configuration of a neutral oxygen atom must account. Previously we discussed the concept of electron shells, subshells,. Web this means that the electron configuration of a neutral oxygen atom must account for 8 electrons. In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. Web therefore the o electron configuration will be 1s 2 2s 2 2p 4. Web to find the electron. Web if we look at the element after nitrogen in the same period, oxygen (z = 8) its electron configuration is: In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. Web the easiest way to create electron configurations is using an electron configuration table, which is a way of writing down the various orbitals available to electrons. Web this means that the electron configuration of a neutral oxygen atom must account for 8 electrons. Web electron configurations are an organized means of documenting the placement of electrons based upon the energy levels and orbitals groupings of the periodic table. 1s 2 2s 2 2p 4 (for an atom). Look at the periodic table and find an atomic number of oxygen, which is 8. The configuration notation provides an easy way for scientists. Electron configurations list every subshell for an atom or ion and how many electrons are in each subshell. Subshells are described by writing the. Remember, a neutral atom contains the same number of protons and electrons. Describe how electrons are arranged in an atom using electron configurations. Web the arrangement of electrons in oxygen in specific rules in different orbits and orbitals is called the electron configuration of oxygen. More specifically, the electron configuration of an oxygen atom. The electron configuration of oxygen. Draw a lewis electron dot diagram for an atom or a monatomic ion.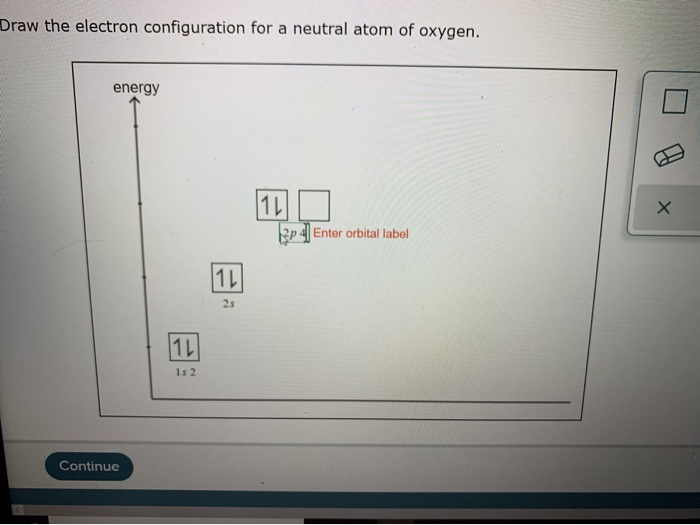
Solved Draw the electron configuration for a neutral atom of

8.2i Writing the electron configuration of a neutral atom with s and p

Diagram representation of the element oxygen Vector Image
Web To Find The Electron Configuration Of Oxygen:
Fill These 8 Electrons In The Following Order:.
Write The Electron Configuration For A Neutral Oxygen Atom And For A Neutral Nitrogen Atom.
Web Consider What Results When A Proton, H+, Becomes Bonded To Methanol By Way Of One Of The Unshared Electron Pairs On The Oxygen Atom, I.e., This Time We Use The Curved.
Related Post: